Keywords
heart failure, fatigue, self-management, systematic review
Fatigue is a common symptom of heart failure which can be distressing for patients and negatively impact both their quality of life and prognosis. We report the efficacy of self-management strategies for people with heart failure-related fatigue.
We searched the MEDLINE, Psychinfo, Emcare and Cochrane Central Register of Controlled Trials (CENTRAL) databases from inception to August 2021 for relevant trials. Twenty-two papers were included describing 21 trials (15 RCTs), comprising 515 participants. Definitions of interventions are given and were grouped as either supported self-management or self-management interventions. Supported self-management included education and person-centred care interventions (n=5). Self-management interventions included mind-body therapies (10), and diet and supplements (6). The Cochrane risk of bias did not show significant high risk across the domains, however the number of participants recruited was small (515 participants in total). There was heterogeneity in intervention type, delivery and outcome measures preventing meta-analysis. Evidence for supported self-management interventions involving education and a person-centred approach, and self-management interventions such as CBT, mindfulness, and some supplements for heart failure-related fatigue is positive, but is limited to individual, small trials. Only eight trials provided a definition of fatigue, and 11 types of fatigue outcome measures were used.
The evidence base for the efficacy of supported self-management and self-management interventions for alleviating heart failure-related fatigue is modest in both study number, size, and quality. Further well-designed trials are needed, along with consensus work on fatigue definitions and reporting.
heart failure, fatigue, self-management, systematic review
Fatigue is a common symptom affecting people with heart failure (HF) and can have a negative impact on their quality of life1. For patients, the sensation of fatigue can be difficult to describe. Words like ‘exhausted’ and ‘tired’ can be used in place of fatigue. There is no universal definition of fatigue, and it can encompass both physical and psychological symptoms2. The pathology of fatigue is related to the widespread activation of stress systems within the body when the heart is under strain3. The New York Heart Association (NYHA) classification system attempts to quantify the degree of limitation from HF symptoms such as breathlessness and fatigue experienced by a patient, but there is no similar scale for fatigue alone currently in use in clinical practice4.
A James Lind Alliance priority setting partnership identified the importance of self-management approaches, and the burden of fatigue for people with advanced HF and the challenges for health professionals to support them5. HF medications and rehabilitation are effective in improving quality of life for people with all stages of HF6. Self-management strategies are increasingly used in healthcare in addition to standard medical therapies7. It is important to distinguish between types of self-management. Guided by National Health Service UK definitions, we define supported self-management interventions as the ways that health and care services encourage, support and empower people to manage their ongoing physical and mental health conditions themselves, and self-management interventions as empowerment of people to manage their ongoing physical and mental health conditions themselves8. These approaches can be powerful adjuncts to traditional therapies for symptom control9. In this systematic review, we aimed to report the efficacy of supported self-management, and self-management interventions for people with HF-related fatigue.
Our overarching review question was, what self-management interventions are used to help patients manage their symptoms of HF-related fatigue and which are most effective? Our Prospero registered protocol is freely available10. The review is reported according to PRISMA guidance using the PRISMA checklist.
Population: adults aged 18+ with chronic HF.
Intervention: any supported self-management or self-management approach aimed at helping people with HF to manage fatigue, e.g., physical activity, nutritional or herbal therapies. We excluded formal cardiac rehabilitation as it is comprehensively reviewed elsewhere11. We excluded treatments requiring a prescription by a medical practitioner.
Comparators: Usual care; comparative studies.
Outcomes: Primary: fatigue measured as a single entity or a component of a measure as self-report or with a formal measurement tool by a health professional. Secondary; safety, adverse events, adherence. We also recorded any given definitions of fatigue and the outcome measure used.
Design: Randomised controlled trials (RCTs); non-randomised controlled trials.
MEDLINE, Psychinfo, Emcare (via OVID) and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched in July 2020 and updated in August 2021. The search was developed for MEDLINE and adapted for the other databases. [Search strategy available on request from authors] Authors of papers included at the full paper stage were contacted, and forward and backward reference searches of included papers were performed. There were no restrictions on basis of language.
The titles and abstracts of references were independently assessed for inclusion by two reviewers (LD, ALH). Disagreements were resolved through consultation with a third reviewer (RJ, BS). For studies of potential relevance, full papers were assessed in the same way. This process was facilitated by Rayyan12.
The data extraction table was piloted with 10% of the included studies (LD, ALH) and adjusted as necessary. Data extraction was undertaken by LD, checked by AH, and discrepancies were discussed.
Quality appraisal of randomised controlled trials and non-randomised controlled trials was conducted using the Cochrane risk of bias tool within Review Manager software (Version 5.4.1)13. The trials were appraised by ALH, checked by LD with discussion as needed.
A meta-analysis for each intervention type was planned for the primary outcome of fatigue14. However, the data were not sufficiently homogenous for meta-analyses, varying in intervention, intensity, duration, fatigue outcome measure and timepoints measured. To give a visual presentation of fatigue data from individual trials, forest plots are presented with data from individual trials, grouped per type of self-management but without meta-analysis. The results of individual trials are reported as calculated mean differences in fatigue between intervention and control groups at the trial endpoint.
Twenty-two papers describing 21 trials were included15–36. [Figure 1]. Fifteen were RCTs, three were pilot RCTs, one was a controlled trial, and two were pilot controlled trials. [Table 1] Interventions were grouped either as supported self-management or self-management. Supported self-management interventions included education and person-centred care (n=5)15–19. Self-management interventions included mind-body therapies (n=10)20–30, and diet and supplements (n=6)31–36.
The mean age was ≤60 years in six trials16,23,24,27,30,32, and 61–75 years in a further 12 trials17,18,20–22,25,26,28,29,31,33,35,36. Only one trial recruited participants between 76–81 years19, and two trials gave age ranges from 30–76 years15,34.
Women were not represented equally, with 14/21 trials having a mean of <50% women participants16,18–23,26,27,30,32–35, of which 10 of the 14 trials recruited <40%16,18,19,21,22,27,30–34, and 5 of the 14 recruited <25% women16,21,22,30,32,33.
NYHA status at recruitment was reported in most trials, and most participants were reported to be in NYHA classes I-III. Two trials focused on classes I-II20,24, five trials focused on classes I-III18,23,30,31,34, one trial focused on classes II-III26 four studies focused on class II only22,33,35,36, four studies focused on classes II-III17,21,25,28,29, and two studies focused on classes III-IV32,34. When NHYA status was not reported, other descriptors were mean ejection fraction % (n=1)27, and time since HF diagnosis (n=2)15,16 In the remaining trial the outcome of fatigue was addressed in a sub-analysis, with only patients who had answered at least one dimension of the Multidimensional Fatigue Inventory 20 questionnaire at baseline being included19.
The 21 trials were conducted worldwide although the USA was the most common location (7). Other locations were Iran (3), Sweden (3), Germany (2), Taiwan (2), and one trial in each in Italy, Mexico, Israel, and Hong Kong.
The number of participants recruited to each trial was small, with a total of 515 recruited across the 21 trials. Seven trials recruited less than 50 participants17,22,25,26,30,32,33.
Recruitment was mostly via hospital outpatient clinics (12/21)17,20–24,26,28–31,33,34. Five trials recruited from hospitalised patients15,16,19,25,27, one trial recruited from both in and outpatients18. One trial recruited from a heart transplant list32 and another recruited via both secondary and primary care35. In the remaining study it was not clear how participants were recruited36.
Eleven different outcome measures for fatigue were used in the trials. [Table 2] These included simple Likert scales for recording of symptom/signs, fatigue specific measures and fatigue subscales of more complex outcome measures. Definitions of fatigue were present in 11/21 trials. Fatigue was described in both physiological and psychological terms across the definitions, but some definitions were very brief. Eleven trials measured fatigue as a standalone outcome; and in 7 of these, fatigue was the primary outcome.
Three of the trials were not randomised and so were graded at high risk of bias for the two domains concerning randomisation and allocation concealment22,28,29,33. [Figure 2] All the 15 RCTs described the random sequence process, 9/15 described allocation concealment16,20,23,27,30–32,35,36.
Most trials were not suitable for participant and personnel blinding, but four trials did, three were supplement trials, the other the mindfulness trial (personnel blinding)26,32,34,35. Only eight trials reported on the blinding of outcome assessment18,23,25,27,30–32,34. Information regarding attrition and reporting bias was well reported. Whilst 11/15 RCTs and one pilot RCT recruited successfully on a sample calculation, all trials lost some participants to follow up15–21,23,24,27,34,35. Two trials had significant attrition21,26. Only three trials described a predefined analysis plan16,19,23.
The evidence for education and support and its impact on the fatigue of people with HF comprises five RCTs15–19. [Table 1]
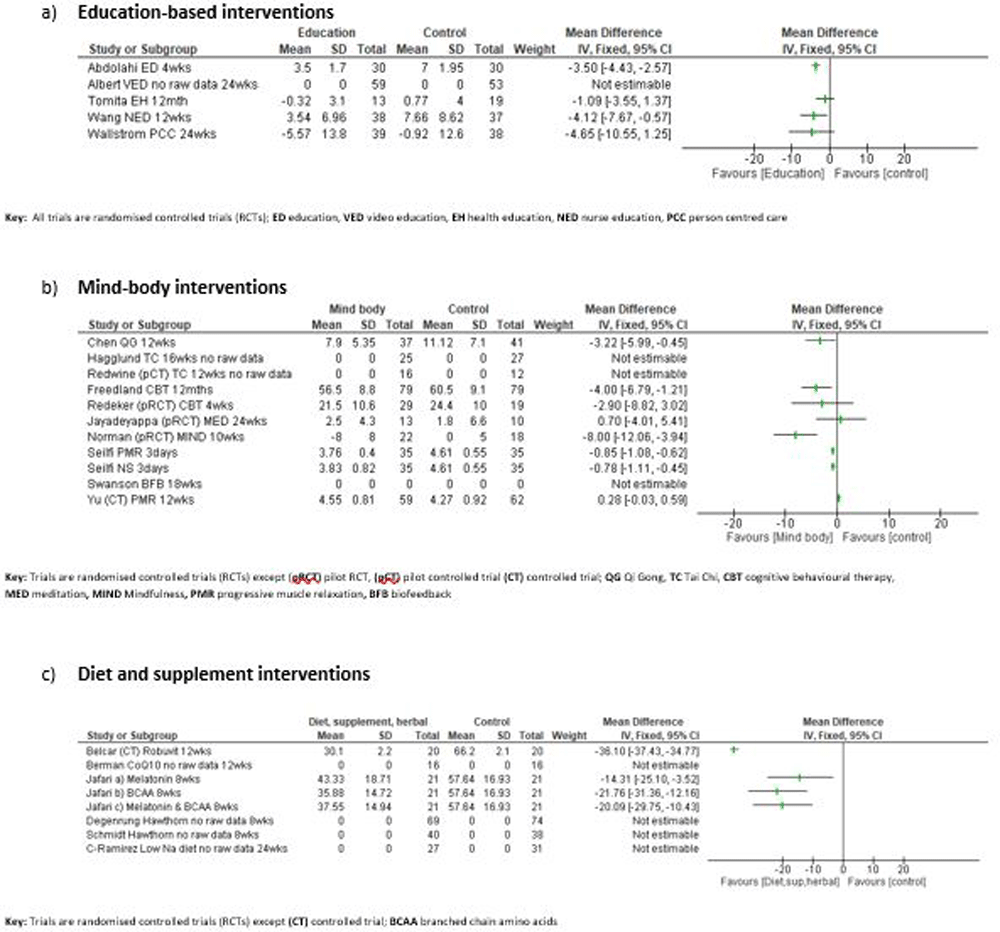
In an RCT by Abdolahi describing four weeks of education compared to regular hospital outpatient care, a statistically significant mean change in fatigue (Piper Fatigue Scale) was reported in favour of the intervention (Calculated MD -3.50 [95% CI -4.43, -2.57]15. An RCT by Albert and colleagues compared a video education package to standard HF education but did not provide sufficient data to examine the effect on fatigue16. The authors reported decreased fatigue (check list with Likert scale) at three months with the intervention compared to usual care (p < 0.01). [Figure 3a]
Mean differences in fatigue outcome between intervention group and control group at end of trial.
The remaining three RCTs on e-health education, supportive educational nursing care and patient centred care reported non-statistically significant improvements in fatigue compared to control groups (Chronic HF Questionnaire, Piper Fatigue Scale, Multidimensional Fatigue Inventory respectively) (Calculated MD 95% CI: -1.09 [-3.55,1.37], -4.12 [-7.67, -0.57], -4.65 [-10.55,1.25] respectively)17–19. [Figure 3a] There were no adverse events reported with these RCTs.
Mind-body therapies. The evidence for mind-body therapies comprises five RCTs, four pilot trials, of which two did not randomise participants and one controlled trial22,28,29. [Table 1] This includes one RCT of Qigong and two trials of Tai chi, two trials (one unrandomised) of Cognitive Behavioural Therapy (CBT), and one trial each of meditation, mindfulness, progressive muscle relaxation (unrandomised), nature sounds and biofeedback20–30.
The RCT conducted by Chen investigated Chinese Qigong versus usual care alone for 12 weeks20. They reported a statistically significant improvement in fatigue (Shortened Piper Fatigue Scale) compared with the usual care (Calculated MD -3.22 (95%CI -5.99, -0.45). [Figure 3b]
The pilot RCT and RCT of Tai chi did not show a statistically significant impact of the intervention on fatigue (both used the Multidimensional Fatigue Inventory)21,22. The pilot RCT authors reported no significant difference between groups at 12 weeks (p =0.19)22. The RCT authors reported that the usual care group had more mental fatigue than the intervention group at six months (p=0 .048)21. The authors were contacted to request further data, but no response was received.
Both trials of CBT reported a statistically significant reduction in fatigue (PROMIS, Multidimensional Assessment of Fatigue) at eight weeks and six months respectively, but only the RCT showed a statistically significant mean change compared with the control group [Calculated MD 95% CI: -2.90 [-8.82,3.02]; -4.00 [-0.679, -1.21] respectively)23,24. [Figure 3b]
A pilot RCT by Jayadevappa compared transcendental meditation with a control group of listened to music or read twice daily25. The authors reported no statistically significant difference in vitality (SF-36 scale) between the groups at six months. (Calculated MD 0.70 [95% CI -0.41,5.41]). [Figure 3b]
A further pilot RCT by Norman and colleagues described a mindfulness intervention which showed a statistically significant improvement in fatigue (Fatigue Severity Scale) compared to usual care alone at 10 weeks (Calculated MD -8.00 [-12.06,3.94])26. [Figure 3b]
A three-arm RCT by Seifi and colleagues compared Benson muscle relaxation (BMR), nature sounds (NS) and usual care for hospital inpatients over three days27. The authors reported an overall statistically significant improvement in fatigue (Fatigue Severity Scale) for all groups. (Calculated MD 95% CI -8.00 [-12.06, -3.94], -0.85 [-1.08, -0.62] respectively). [Figure 3b]
In the controlled trial by Yu published over two papers, one of the trial groups recieved a progressive muscle relaxation intervention28,29. The authors reported a non-statistically significant improvement in fatigue (Chronic HF Questionnaire) compared to the control group at 12 weeks [Calculated MD 0.28 [95% CI -0.03, 0.59]. [Figure 3b]
An RCT by Swanson investigated cardiorespiratory biofeedback training versus sham biofeedback over six weeks30. The authors did not provide sufficient data to examine but reported that there was no significant difference in fatigue (Borg Scale) between the groups. [Figure 3b]
No adverse events were reported in four of the trials20–23 and a further four did not make a statement24,25,27–29. The mindfulness trial reported an adverse event based on the statement of one participant who dropped out reporting a ‘gloomy mood’ related to increased bodily symptom awareness26.
Diet and supplements. The evidence for diet and supplements comprises five RCTs and one pilot controlled trial31–36. One RCT investigated a low salt, balanced dietary regime31. Three trials looked at dietary supplements: oakwood extract, Co-enzyme Q10, melatonin (Me) and branched chain amino acids (BCAA)32–34. The two remaining RCTs investigated the herbal supplement hawthorn (Crataegus)35–36.
There were insufficient data reported in the trials of low salt (only p values given), CoQ10 (no intergroup comparisons) and hawthorn trials (general statements) to determine any impact of the interventions31,32,35,36. [Table 1].
A controlled trial by Belcaro investigated Robuvit® (Oak wood extract) compared to usual care alone. Participants in the Robuvit® group had a statistically significant reduction in fatigue (Multi-dimensional Assessment of Fatigue scale) compared to controls at 12 weeks (Calculated MD -36.10 (95% CI -37.43, -34.77)33. [Figure 3c]
In a four-arm RCT by Jafari-Vayghan the impact of Me and BCAA supplementation, separately and together with an identical placebo group were investigated34. All three supplement groups showed a statistically significant reduction in fatigue (Fatigue Symptom Inventory) compared to the placebo group at eight weeks (Calculated data M: MD -14.31 [95% CI -25.10, -3.52] BCAA: MD -21.76 [-31.36, -12.16], M & BCAA: MD -20.9 [-29.75, -10.43]) [Figure 3c]
Two RCTs did not report adverse events31,34, and the Coenzyme Q10 RCT reported gastrointestinal upsets with the supplement but did not provide any numbers32. The two hawthorn RCTs gave detailed adverse events reporting as it was a primary outcome, but none of the serious adverse events were thought to be caused by the supplement35,36. The Robuvit® trial reported that no side effects were observed33.
This systematic review includes 21 trials investigating the efficacy of a variety of supported self-management, and self-management interventions for HF-related fatigue. A recent James Lind Alliance research prioritisation partnership for advanced HF highlighted the importance of fatigue to people with HF and supported the need for these types of interventions alongside medication and cardiac rehabilitation5. UK and European guidance suggests that a personalised, exercise-based cardiac rehabilitation programme should be offered to all people with HF, unless their condition is unstable6,37. Furthermore, this provision should include psychological and educational content coupled with ongoing support6,37. Many of the interventions included in this systematic review are complementary to the recommendations of this guidance.
The supported self-management interventions in this review focused on education, support, and in one RCT, person-centred care15–19. Limited data for this type of intervention suggests a short term positive effect on fatigue, diminishing overtime. Related research in education for people with HF suggests that family-based education is preferable, but these studies do not identify fatigue as a standalone outcome38. Future research into the role of education should focus more on the important outcome of fatigue.
The self-management interventions in this systematic review formed two broad groupings of mind-body, and diet and supplements.
The group of mind-body therapies covered a range of interventions from CBT to biofeedback20–30. Of note was the RCT of CBT showing a statistically significant effect on HF-related fatigue at 12 months23. The pilot RCT of mindfulness also showed a statistically significant effect on HF-related fatigue at 10 weeks26. These findings are supported by a recent systematic review of psychological interventions including CBT for HF which suggested a short term (<3 months) effect of a variety of psychological interventions on health related quality of life39.
A 2020 systematic review and meta-analysis of the benefits of Tai Chi for HF reported that it improves exercise capacity, quality of life, depression, and decreased b-type natriuretic peptide expression40. However, a more recent overview on the same topic concluded that more robust trials are needed, and that careful consideration is required in meta-analysis of these data41. Indeed, our review shows that fatigue data from the two Tai chi trials were not presented fully to allow proper interpretation.
Five of the seven diet and supplement trials did not provide sufficient detail on the fatigue outcome data. This lack of evidence is reflected in a recent systematic appraisal and evidence map of diet and supplements for people with HF which does not include the outcome of fatigue in its otherwise comprehensive approach42. Data from the Robuvit®, Melatonin and BCAA supplement trials in the current review both reported a statistically significant impact on fatigue in the short term33,34.
A recent systematic review and meta-analysis of Co Q10 supplementation for HF suggested a possible benefit of coenzyme Q10 on all-cause mortality; the results for short-term functional outcomes were more modest or unclear43. Fatigue was not included as a standalone outcome.
The supplement Robuvit® is less researched. The proposed mechanism of action involves the constituent polyphenols working to reduce oxidative stress in the body, and therefore impacting on energy capacity44. The included trial for HF-related fatigue was a small non-randomised controlled trial and requires robust replication33. Melatonin conversely has been investigated for its potential role in cardiovascular disease over the last two decades45. The most recent RCT looking at melatonin supplementation for people with HF reports an improvement in quality of life compared to placebo over 24 weeks46. There is little research on BCAA supplementation, and none of it in people with HF.
Overall, the participants of the included trials of supported self-management and self-management interventions do not represent the general HF population. The prevalence of HF slowly increases with age until about 65 years, after which it increases more rapidly. One in seven people have a diagnosis of HF at 84 years47. Most of the participants in the included trials were under 75 years of age, and a significant number were 60 years or younger. A recent review of HF confirms that most interventional research is conducted with younger participants, whereas older, potentially more frail people with HF are the subject of qualitative studies48.
The female gender is underrepresented in the trials and therefore caution is required when generalising from the results of studies with mostly male participants. A recent systematic review outlines the benefits of women-focused rehabilitation programmes for physical and mental dimensions of quality of life49.
There was no language restriction in the conduct of this review, and the trials included were undertaken in a range of countries and included a range of ethnicities. We are aware that there is a lack of HF data generally from countries outside Europe and North America, especially from lower and middle-income countries, even though these are estimated to carry 80% of the global cardiovascular disease burden50.
People with more severe HF (NHYA III and IV), who are likely to be experiencing greater fatigue, are under-represented in the studies included in this review. Only the CoQ10 and melatonin/BCAA RCTs included this profile of participants, with the former focusing on end stage HF, but the latter including only three participants with a NYHA status IV out of a total of 8432,33.
A further aim of this systematic review was to examine how fatigue was defined and measured in these trials. Only half of the included trials gave a definition, and some of these were brief. This was in part because fatigue was only a primary or standalone outcome in eleven of the trials.
In the HF literature with a focus on physical activity and rehabilitation there is a tendency to focus on acute fatigue and its recovery after exercise exertion. Whilst this is an important area, it does not address the more global physical and psychological fatigue that people with HF experience on day-to-day. Over the 21 trials, eleven distinct fatigue outcome measures were used, from unvalidated, simple Likert scales based on symptoms, to fatigue items in established composite HF outcome measures. Further research is needed to be able to assess and quantify the patient experience of fatigue, potentially through the development of well-validated patient-reported outcome measures.
In conclusion, there is a limited evidence base for supported self-management and self-management interventions for HF-related fatigue. However, these interventions are complementary to conventional HF management and may be of value if rehabilitation is not available or not appropriate. Supported self-management approaches involving education and ongoing support are likely to help people with HF generally in their quality of life, including managing fatigue despite the modest, short-term evidence. The efficacy evidence for a variety of self-management interventions such as CBT, mindfulness, and some supplements for HF-related fatigue is positive, but is limited to individual, small trials. All promising trials within this systematic review warrant replication.
In addition, this systematic review highlights two important issues associated with HF research. Firstly, that participant recruitment into supported self-management and self-management trials does not represent the real-world HF population in terms of age, gender, ethnicity and severity of disease. Secondly, it highlights the lack of focus of research on the everyday fatigue people with HF live with.
What is needed now is the acknowledgment of the importance of fatigue to people with HF, a better understanding of the impact of fatigue on quality of life, and more research into the promising interventions.
Further well-designed trials are needed to provide more robust evidence for self-management strategies for people with HF-related fatigue. These trials need to be designed to better reflect the characteristics of the HF population. There is also a need for consensus work on fatigue definitions and outcome measures to allow clear and standardised reporting in future studies.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: Appendix 2 Medline search strategy: systematic review of self-management of heart failure-related fatigue, https://doi.org/10.6084/m9.figshare.21572856.v151.
Figshare: Appendix 3, intervention definitions: systematic review of self-management of heart failure-related fatigue, https://doi.org/10.6084/m9.figshare.21572889.v152.
Figshare: Appendix 1 PRISMA checklist: systematic review of self-management of heart failure-related fatigue, https://doi.org/10.6084/m9.figshare.21572700.v153.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Psychosocial oncology, behavioral symptom management interventions
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Adult Health Nursing: Oncology, Wound Care, Quality of Life Issues, Cardiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 19 Dec 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)