Keywords
COVID-19, Long-COVID, paediatrics, infectious diseases
Children and young people (CYP) may experience prolonged symptoms following COVID-19, commonly termed ‘Long-COVID’. The characteristics of Long-COVID in CYP are unclear, as are the sequalae of acute COVID-19. We aimed to systematically synthesise evidence of the long-term outcomes of COVID-19 in CYP.
13 electronic databases were searched until January 2022. Inclusion criteria: observational studies reporting outcomes occurring four-weeks or more after COVID-19 in children <18 years old. Exclusion criteria: outcomes of Paediatric Inflammatory Multisystem Syndrome. Title, abstract and full text screening were conducted independently by two reviewers. Data extraction and risk of bias assessment was by one reviewer with independent verification. Critical appraisal tools appropriate for study type were employed. Results were narratively synthesised with meta-analysis to generate summary estimates of risk of prolonged symptoms in CYP.
94 studies were included in this systematic review. Of these, 66 studies recruited from hospital settings and 8 studies recruited solely from community settings. Over 100 symptoms were reported, the most common being fatigue, headache and cognitive symptoms. Summary estimates of prevalence of prolonged symptoms were higher for hospital samples (31.2%, 95% CI 20.3% to 43.2%) than for community samples (4.6%, 95% CI 3.4% to 5.8). Reported sequalae of COVID-19 in CYP included stroke, type-1 diabetes, Guillan-Barre syndrome, and persistent radiological or blood test abnormalities. Most studies reporting these sequalae were case reports / case series and the quality of evidence in these studies was low.
Prolonged symptoms following COVID-19 in children are variable and multi-systemic. Rates of prolonged symptoms in community samples are lower than hospital samples. There is currently limited good quality data on other sequalae in CYP. Heterogeneity in methods of diagnosis of COVID-19, symptom classification, assessment method and duration of follow-up made synthesis less secure.
For most children and young people (CYP), COVID-19 is a mild illness. Some children however, get symptoms that last a long time (Long-COVID) or have other health problems following COVID-19. There are still unanswered questions about the longer-term effects of COVID-19 in CYP. We set out to summarise the evidence published so far to help understand what support and further research is needed for affected CYP.
We searched electronic databases for published research about COVID-19 in CYP. We picked out studies that described any effect that lasted longer than four weeks. We did not look at studies focussed on a complication of COVID-19 called PIMS-TS as this is a rare and separate illness. We summarised the findings of the studies and looked at whether the research was done in a way that meant we could be confident in the results.
We included 94 studies. Over 100 different symptoms that lasted more than four weeks were reported. The most common symptoms were fatigue, headache and things like ‘brain fog’. Most studies included CYP who had been to hospital. Higher numbers of CYP in these studies had long-lasting symptoms than in studies that only included CYP from community settings. Only five studies included a group of CYP who didn’t have COVID-19. The results from these studies showed that CYP with COVID-19 were more likely to have symptoms lasting more than four weeks than those who didn’t have COVID-19 or had other viral illnesses.
Some CYP developed other long-lasting health problems after having COVID-19, including strokes and diabetes. Most reports of these types of problems included small numbers of CYP and although these are serious problems for those CYP who are affected, it is hard to be certain how big a problem this is for society and for healthcare services.
COVID-19, Long-COVID, paediatrics, infectious diseases
Acute SARS-CoV-2 infection (COVID-19) in children and young people (CYP) typically presents as a mild illness resulting in fewer hospitalisations, complications, or deaths than in adults1. Fever and cough are the most common symptoms, with other symptoms including rhinorrhoea, sore throat, headache, fatigue/myalgia and gastrointestinal symptoms occurring in less than 10–20%2.
It became evident early in the pandemic that some people experience prolonged symptoms following COVID-193,4. Definitions of this condition vary within the literature5,6 although the patient derived term7, Long-COVID, is well-established. The National Institute for Health and Care Excellence (NICE) use the clinical case definitions of ongoing symptomatic (or post-acute) Covid-19 (5–12 weeks after onset) and post-Covid-19 syndrome (symptoms lasting 12 weeks or more)8 whilst acknowledging that ‘Long-COVID’ is commonly used to encompass both definitions. Research into Long-COVID has predominantly focused on adults and literature on the condition in CYP is more limited9,10.
Three reviews have reported clinical presentation and prevalence of Long-COVID in CYP11–13, though their findings range from no difference in persistent symptoms between cases and control groups11, to a higher risk of some persistent symptoms in cases compared to controls12, and a narrative synthesis finding that prevalence estimates and symptom burden varied widely13. Reported ‘prevalence’ in these reviews referred to risk of prolonged symptoms in children who had had COVID-19, rather than population prevalence.
Studies in adult populations have demonstrated increased risk of a variety of complications following COVID-19, including cardiovascular events, strokes and venous thrombosis14–17, with greater risk of severe COVID-19 and complications in people with underlying chronic conditions18. These areas have not been well studied in CYP.
Identifying the most common symptoms and sequalae that CYP experience following COVID-19 and estimating their frequency will help clinicians to recognise these complications and implement personalised management. Such information is also necessary to inform development and commissioning of appropriate services. We aimed to synthesise knowledge of longer-term effects of SARS-CoV-2 infection on CYP. Specific objectives were to identify; 1) patterns of symptoms lasting longer than four-weeks 2) risk of developing prolonged symptoms, 3) other sequalae of the infection (including clinical effects and persistent radiological and pathological findings) and 4) longer-term effects in children with pre-existing long-term conditions.
This review is part of a larger programme of work investigating symptom patterns and life with longer-term COVID-19 in children and young people (SPLaT-19), which includes a prospective cohort and nested qualitative study (https://www.keele.ac.uk/ctu/researchportfolio/activeresearch/splat/#!). Whilst there was no patient and public involvement (PPI) work specifically informing this systematic review, the wider project was developed with input from children and young people and has PPI embedded at all stages.
This systematic review is registered with PROSPERO (Registration number CRD42020226624) and is reported in accordance with Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) 2020 guidelines)19. We included studies published between December 2019 and January 2022 that reported outcomes at four-weeks or beyond in children aged 0 to 18 years old who had had COVID-19 (positive antigen test, or clinical diagnosis of COVID-19). Pre-prints were included if they were published in a peer-reviewed journal before November 1st, 2022.
Studies were excluded where duration of symptoms was unclear, or less than four-weeks, although studies reporting development of new chronic morbidities secondary to COVID-19 infection were included, on the basis that these morbidities would persist beyond four weeks. Studies including adults were excluded unless data pertaining to children were separately presented. Studies solely reporting data on children affected by Paediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS) were excluded, given the unique nature of this rare and severe complication. Primary research of any design, any setting and in any language was included. Secondary evidence, conference abstracts and trial protocols were excluded.
We searched 13 electronic databases (MEDLINE, EMBASE, AMED, HMIC, CINAHLPlus, PsycINFO, Web of Science (Science Citation and Social Science Citation indices), ASSIA, WHO COVID-19: Global literature on coronavirus disease, Cochrane COVID-19 study register, ProQuest Coronavirus research database, NDLTD and OpenGrey) and reference lists of existing systematic reviews. Searches utilised text word searching in the title, abstract and keywords, along with database subject headings, combining terms for paediatrics AND COVID-19 AND long-term. Search terms were adapted for each database platform.
Searches were run by an information specialist (NC), up to 31st December 2021. Results were imported into Endnote X9 (reference management software, Clarivate Analytics, available at https://endnote.com/) where duplicates were removed. Unique references were uploaded to Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org) to manage the screening process.
Title and abstract and full text screening were conducted independently by two reviewers (from CB, HT, VW, GS, TR). Discordance was resolved by team discussion (CB, NC, HT, VW, GS, TR). Main reasons for exclusion during the full text stage were recorded.
Data extraction and risk of bias assessment was carried out by one reviewer, and independently checked and validated by a second. We extracted the following data: author; year of publication; setting (country of origin, specific details of community, healthcare setting); study type; study aim; funding; age of participants (range, mean, SD); sex (counts and proportions); number of participants; COVID-19 exposure: definition (including symptom description, antigen test status); details of any comparator group; description of any covariates (e.g. demographic information); potential prognostic factors; outcome measure(s): which type were measured, how they were measured; follow-up time points; measures of association where relevant.
Risk of bias was assessed using the relevant Critical Appraisal Skills Programme (CASP) checklist (https://casp-uk.net/casp-tools-checklists/) for cohort studies and the Joanna Briggs Institute critical appraisal tool (https://jbi.global/critical-appraisal-tools) for case studies and case series. Results of the checklists were used to categorise studies as high, moderate, or low risk of bias.
Studies were grouped according to the four objectives; those that reported symptoms lasting more than four-weeks (objectives 1 and 2), those reporting sequalae of the acute infection persisting for more than four-weeks (including new clinical diagnoses and persisting pathological or radiological abnormalities in asymptomatic children) (objective 3), and those reporting prolonged symptoms in children with pre-existing long-term conditions (objective 4).
The method of analysis varied for the different objectives. To describe patterns of symptoms (objective 1), where it was possible to discern numbers of children affected by a particular symptom, symptoms were grouped into clinical categories and an infographic was developed to present findings in a clinically meaningful way. To estimate the risk of prolonged symptoms in CYP who have had COVID-19 (objective 2) a meta-analysis was carried out. For objectives 3 and 4, findings were narratively synthesised20 but due to heterogenous research questions, study types and outcomes, no quantitative synthesis was attempted. When summarizing findings, issues related to study limitations (risk of bias), precision (small samples), inconsistency of results, or generalisability were highlighted.
The meta-analysis included studies which reported the proportion of children with COVID-19 who experienced prolonged symptoms to generate summary estimates of the risk of prolonged symptoms in CYP following COVID-19. Risk was calculated using the numerator as the number of participants with symptoms lasting longer than four-weeks and the denominator as the total number of participants. The analysis was stratified by recruitment setting (hospital or community) and further by duration of prolonged symptoms (beyond four or twelve weeks) as appropriate.
Individual study prevalence (risk of prolonged symptoms) was pooled using inverse variance DerSimonian and Laird method to fit the random effects model, selected due to the methodological variability across studies. Heterogeneity was summarised using the estimate of between study variance (t2), and the proportion of variability in effect estimates due to between study heterogeneity was summarised using I2. A 95% prediction interval for the random-effects model was calculated if at least three studies were available in a meta-analysis21. Where comparator group data were available, we calculated pooled odds ratio (OR) and 95% CIs using the same methodology.
Publication bias was assessed by visual inspection of a funnel plot if ten or more studies were available for a given outcome. Asymmetry in the publication bias was tested by Egger’s method (p<0·1 was considered as an indication of publication bias)22. Meta-analysis and subgroup analysis were performed using Stata v17·0 (Stata-Corp, College Station, Texas, USA). A p-value <0·05 was considered statistically significant for overall and subgroup effects.
The study selection process is represented in Figure 1. 94 studies were included in the review23–116. Characteristics of included studies are summarised in Table 1. Data extracted from all included studies and risk of bias judgement for each are summarised in Table 2.
Included studies came from 47 different countries although the majority were from Europe (43 studies)24,28–32,34,36,39,40,44,49,50,52,53,55,58–60,68,74,75,77–79,81,82,84,88–91,94–96,98,103,104,107,113,114, and the USA (26 studies)33,35,37,41–43,45–48,57,61,63,67,69–71,76,83,86,93,99,109–112. Articles were translated from Norwegian, Russian, and Mandarin. 38 were case reports23,33,36,41,43,47,48,51,52,55,57,60–62,64,66–70,72,78,80,85–87,89,92,98–101,107,108,110,112. Of those that were not case reports, most were cohort studies (24 studies)29,30,37,39,40,44,50,53,54,56,63,76,81,82,88,90,91,94,95,97,103–105,114. Most studies recruited from hospital or secondary care settings and only eight recruited solely from community settings38,81,82,90,91,94,107,114.
Most studies only included participants who had had a positive antigen test for Sars-CoV-2, although 16 studies included participants where diagnosis was either clinical or by self-report31,35,42,70,73,74,75,80,81,83,98,102,106,113,115,116. The longest duration of follow up was 10–13 months79. 36 studies were judged to have low risk of bias23,27,28,32,33,36,38,41,46,48,51,52,55,58,59,64–66,69,71,73,76,78,81,83,85–87,89,94,98,100,109,110,112,114, with 19 studies in the high-risk of bias group25,30,35,39,47,53,54,57,62,75,77,79,80,92,99,102,105,111,115. Of 24 cohort studies, 15 were judged as moderate risk of bias29,40,44,50,56,63,82,88,90,91,95–97,103,104. The commonest bias risks were the identification and handling of potential confounding factors, and lack of generalisability, often due to small sample size. The four cohort studies deemed low risk were large, matched cohort studies37,81,94,114. Of 25 case series25,27,28,31,32,34,35,42,46,59,71,73–75,77,79,83,93,102,106,109,111,113,115,116, a third were judged high risk of bias25,35,75,77,79,102,111,116, a third moderate risk of bias31,34,42,74,93,106,113,115 and a third low risk of bias27,28,32,46,59,71,73,83,109. Lack of detail on completeness of inclusion and the clinical setting and or demographics of participants were the commonest bias risks.
Where symptoms of COVID-19 continued for more than four-weeks, over 100 symptoms affecting multiple body systems were reported (Figure 2). Fatigue was reported in the highest number of studies (478 participants out of 1192 with prolonged symptoms in 23 studies), followed by headache (192/1121/18) and cognitive symptoms (248/949/15). After constitutional and neurological symptoms, the most affected systems were respiratory, ear, nose and throat, and musculoskeletal.
There were 17 studies26,30,32,53,56,74,79,81,82,88,91,94,95,97,103,104,114 (Table 3) that reported numbers of participants with prolonged symptoms from a defined population of participants with COVID-19 and these were included in a meta-analysis. Of these, 11 recruited from hospital settings26,30,32,53,56,79,88,95,97,103,104 and six recruited from the community74,81,82,91,94,114. There were 14 cohort studies26,30,53,56,81,82,88,91,94,95,97,103,104,114, and three longitudinal case series32,74,79 (all three case series contained at least 70 participants).
Summary estimates of risk of prolonged symptoms showed large variability but were higher among hospital samples (33.9%, 95% CI 21.5% to 47.4%) than in community samples (5.1%, 95% CI 3.6% to 7.0%) (Figure 3). Studies were stratified by those reporting symptoms beyond 12 weeks and those reporting symptoms between 4 and 12 weeks. Studies with longer follow-ups reported higher rates of prolonged symptoms (27%, 95% CI 13·3% to 43.2%) than those with short follow-ups (14.6%, 95% CI 8.1% to 22.6%) (Figure 4). In these subgroup analyses, the estimate of between study heterogeneity (τ2) varied from 0·111 (studies recruiting from community populations) to 0·224 (studies reporting symptoms beyond 12 weeks).
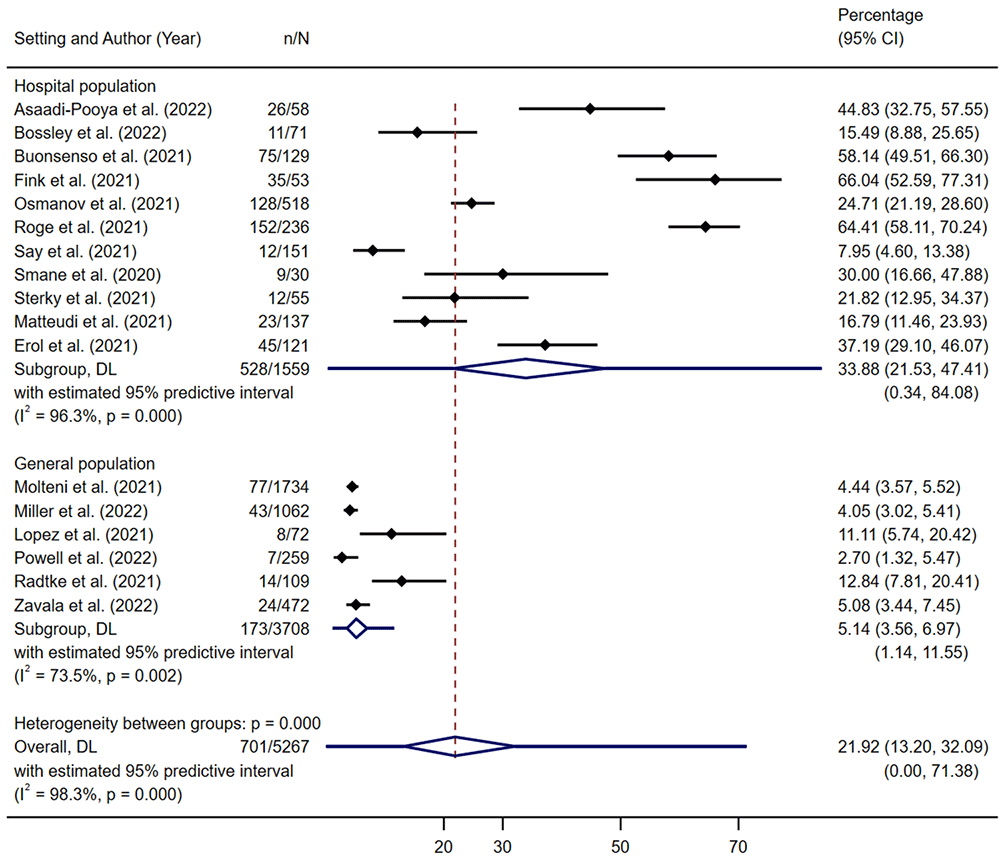
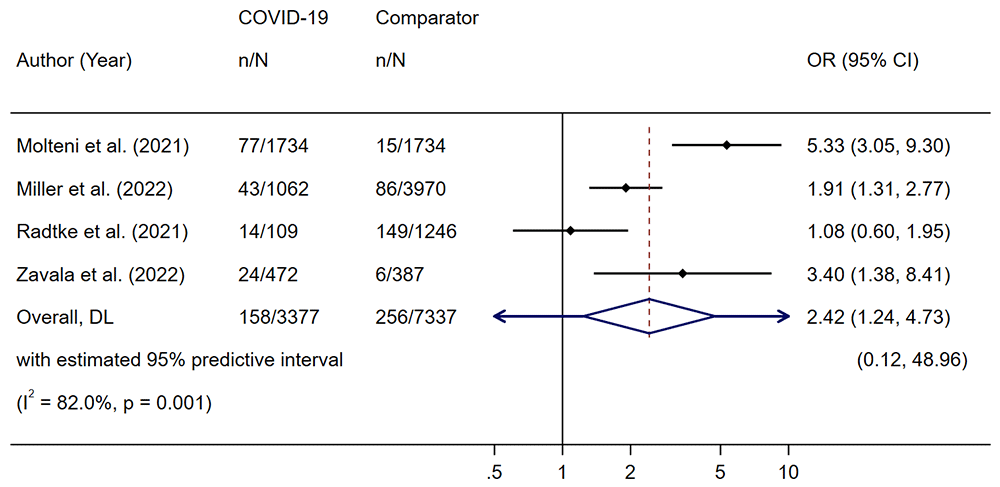
Five studies reported comparator data81,82,94,95,114. Four of these were set in the community and had controls who were either sero-negative or had no evidence of past infection81,82,94,114. One study recruited from a post-acute hospital clinic (participants included both those hospitalised and non-hospitalised for the acute illness) and the control group was comprised of children with other viral illnesses95. In the community studies, the estimated summary OR of prolonged symptoms in the COVID-19 positive group compared to the non-COVID group was 2.4 (95% CI 1.2 to 4.7) (Figure 5), indicating higher likelihood of prolonged symptoms in CYP with COVID-19 compared those without COVID-19. This result is subject to substantial between-study heterogeneity (τ2 0.372, I2 82%, P<0.001). We assessed publication bias for risk of prolonged symptoms in the studies recruiting from hospital (the community group contained <10 studies). Funnel plot asymmetry showed potential small-study effects on funnel plot (Figure 6) although this was not statistically significant (β = 5.4, t = 1.36, P = 0.206).
Two cohort studies39,90 reported group level findings rather than individual numbers of participants affected and thus were not included in the meta-analysis. Clavenna39, a matched cohort study judged as being at high risk of bias, reported that the prevalence of symptoms over six months of follow up was similar in those who had had COVID-19 to those who had not had COVID-19. Petersen90 judged as being at moderate risk of bias, reported that children were less likely than adults to experience prolonged symptoms after COVID-19.
17 studies (two cohort studies29,37, four case series71,102,106,109, one cross-sectional study38 and 10 case studies24,47,61,64,65,80,86,87,98,100 described clinical sequalae of COVID-19 occurring after, or lasting more than, four-weeks from the acute infection. One large, matched cohort study (predominantly adults but including 2673 matched pairs of children), judged as having a low risk of bias37, observed all new conditions presenting one to four months after COVID-19, and reported that children who had had COVID-19 were not more likely to develop new conditions than controls.
Stroke was reported as an outcome in four studies: one case series71 and three single case studies65,98,100. The case series, assessed as being of low risk of bias, observed three cases of acute ischaemic strokes, and four cases of haemorrhagic stroke amongst 1695 children hospitalised due to COVID-19.
The association between new-onset diabetes and COVID-19 was considered in five studies. Two studies observed populations of children presenting with new-onset type-1 diabetes for evidence of co-existent SARS-CoV-2 infection. Trieu109 was judged to be of low risk of bias and reported nine cases of COVID-19 in 286 children hospitalised with new onset type-1 diabetes, and one case of COVID-19 in 290 children hospitalised with new onset type-2 diabetes. Boboc29 was judged to be of moderate risk of bias and reported eight children with COVID-19 out of 459 hospitalised with new onset type 1 diabetes. One case series (Thakur & Rai106, n=2), and two case studies86,87 also described cases of onset type-1 diabetes with recent COVID-19.
Steroid-dependant autoimmune haemolytic anaemia was reported in one case from 397 children with confirmed SARS-CoV-2 infection38. Other sequelae reported in case series or case studies included acute pancreatitis with pseudocyst formation102, Guillain-Barre syndrome (3 case studies24,64,80), acute psychosis61 (one 17-year-old), and concurrent rheumatic fever47 (one case study).
Ten studies reported laboratory or radiological abnormalities that persisted for more than four-weeks from COVID-19 infection despite symptom resolution44,57,58,66,68,78,92,99,105,115. Two cohort studies reported persistent abnormal lung imaging in children previously hospitalised with COVID-19 (22 cases in Tang105, n=46, high risk of bias; and ten in Denina44, n=25, moderate risk of bias). A case series (n=14) also reported persisting chest CT abnormalities in seven children115. Retinal vasculature abnormalities were found in children who had COVID-19 in a case-control study (cases=63) deemed low risk of bias58. Six case studies reported other abnormal blood or imaging findings persisting beyond four-weeks, including leucopaenia68,92,99, idiopathic thrombocytopaenia68, raised inflammatory markers44, right coronary artery dilatation on echocardiogram57, and residual spinal MRI changes after clinically resolved Guillan-Barre syndrome associated with COVID-1966.
16 studies (including nine case studies) described the effects of COVID-19 in children with specified underlying conditions and reported prolonged symptoms or complications in at least some of the participants23,27,28,33,42,48,51,59,60,62,67,72,76,89,110,113. The underlying conditions were predominantly associated with immunodeficiency, either due to the primary condition or its treatment.
Only three of these studies were cohort studies or large case series. Madhusoodhan76 (low risk of bias) was a multi-centre study including 98 children with malignancies who tested positive for Sars-CoV-2. The reported range of symptom duration was up to 52 days (median 10) and range of duration of in-patient care for the acute illness was up to 65 days (median 12). Kamdar63 (moderate risk of bias) followed 109 children with haematological-oncological conditions who had COVID-19 and reported only one child with a late complication, although duration of symptoms for most participants was not reported. Conway42 (moderate risk of bias) was a case series of 225 heart transplant candidates or recipients who had COVID-19, in which seven had on-going symptoms at 30 days.
Other studies in this category were small case series or case reports. Most described prolonged COVID-19 illness complicated by the underlying condition or its treatment27,33,48,67,72,89,110,113. Some described complications that were thought to be due to COVID-19 such as myocarditis occurring two months after COVID-19 in a child with renal failure60, right atrial thrombus in a child with acute lymphoblastic leukaemia (ALL)23 and immune thrombocytopaenic purpura occurring 22 days after COVID-19 in a child with ALL51. Others described an effect of COVID-19 on the underlying condition e.g. three children with flares of juvenile idiopathic arthritis occurring or lasting more than four weeks after COVID-1959.
This review describes the spectrum of reported outcomes beyond four-weeks in children who have had COVID-19. The most frequently reported prolonged symptoms were fatigue, headache and cognitive difficulties. Summary estimates of risk of prolonged symptoms were higher in studies recruiting from hospital settings (31.2%, 95% CI 20.3% to 43.2%) than community settings (4.6%, 95% CI 3.4% to 5.8%). Children who had had COVID-19 were more likely (OR 2.96) to experience prolonged symptoms than those who had not. Sequalae including stroke, type-1 diabetes, Guillan-Barre syndrome, and persistent radiological or blood test abnormalities have been reported in CYP following COVID-19 but most studies reporting these are case reports or case series and quality of their evidence is low.
Like previous reviews, we found that where COVID-19 symptoms persist for more than four-weeks in children, they are multisystemic and vary widely between individuals. In alignment with our findings, two previous systematic reviews of persistent symptoms of COVID-19 in CYP11,12 also found symptoms across different systems, with constitutional and neurological symptoms amongst the most commonly reported. This is analogous to studies of adults with Long-COVID where systematic reviews117,118 have found general malaise, fatigue, sleep disturbance and concentration impairment to be commonly reported. In adult populations however, breathlessness and altered sense of smell are often higher up the ranking of symptom frequency14,117–119.
Most studies in our meta-analysis of prolonged symptom prevalence included children recruited from hospital settings. However, stratification by setting showed that risk of prolonged symptoms was higher in studies of children recruited from hospital settings than in studies recruiting from community settings. This is important, given most children with COVID-19 have mild illness and do not require hospitalisation. There have been large community studies published since the search window for this review, which may go some way to redressing this balance. These include the CLoCK study, a matched cohort study of post-COVID-19 condition among children and adolescents 11 to 17 years of age that recruited participants using the British national testing database10 and a further matched cohort study from Germany14 which used routine health care data to report outcomes at least three months after COVID-19.
Rates of prolonged symptoms in the studies included in the meta-analysis were higher in studies that reported outcomes beyond 12 weeks than in those that reported symptoms at four-weeks. This is to be interpreted with caution as the numbers in the studies with longer follow-up were smaller and there was only one study in this group which recruited from a community setting. Whilst both the NICE and United States Centres for Disease Control and Prevention (CDC) definitions of post-COVID conditions encompass symptoms continuing or occurring more than four-weeks after acute infection, the World Health Organisation (WHO) has recently published a consensus definition of Post COVID-19 condition in children and adolescents, which refers to “individuals with a history of confirmed or probable SARS-CoV-2 infection, experiencing symptoms lasting at least 2 months which initially occurred within 3 months of acute COVID-19"120. This review highlights that the evidence base for characterising and understanding symptoms over this duration in CYP is sparse.
Only five studies in our meta-analysis included a comparator group. Given the broader effects of the pandemic and associated social restrictions on children’s health, high quality matched studies are critically important to understanding effects directly attributable to Sars-CoV-2 infection. From the limited data available for analysis, the likelihood of having symptoms lasting more than four weeks is higher in children who have had COVID-19 than in those that have not. Of note, both large community studies mentioned above included a control group. The CLoCK study’s10 first follow-up data showed that children with a positive test were significantly more likely to report multiple symptoms at three months than controls, and Roessler14 found increased rates of physical and mental health conditions in cases than controls, with highest incident rate ratios for malaise, fatigue/exhaustion and cough.
Children with medical complexity and certain underlying conditions have been shown to be at higher risk of severe COVID-19121. The few studies in this review that focussed on children with specific conditions did not demonstrate high numbers of children experiencing long-term effects. However, as there were only three cohorts / case series in this group and they were not specifically designed to study long-term outcomes, firm conclusions cannot be drawn.
Our review is unique in that, alongside pattern and risk of prolonged symptoms, we have synthesised evidence on other sequalae of COVID-19 in CYP and have included evidence down to single case report level. Whilst small case series and individual case studies should not be given undue weight in evidence syntheses, their inclusion in the context of an emerging pandemic and rapid accumulation of knowledge about its long-term consequences is important. The most common conditions reported to have developed in temporal association with COVID-19 were neurological conditions, including stroke and Guillain-Barre syndrome, and type-1 diabetes. In adults, COVID-19 infection has been associated with a range of long-term conditions / complications including new onset diabetes122,123 venous thromboembolism14, neurological complications including stroke124,125 and cardiovascular disease126. The extent to which CYP are affected by similar sequalae is less well characterised. One recent report using US CDC data121 did identify increased risk of acute pulmonary embolism (adjusted hazard ratio = 2·01), myocarditis and cardiomyopathy (1.99), venous thromboembolic event (1.87), acute and unspecified renal failure (1·32), and type-1 diabetes (1.23) in under 18s, but this needs further confirmation.
Synthesis of persisting radiological and pathological findings following COVID-19 in CYP is also novel. A systematic review of long-term effects of COVID-19 in adults looked at persistently abnormal clinical investigations in hospitalised patients and found lasting changes in lung function and structure118 but previous reviews in paediatric populations have not considered this. The frequency, clinical significance and duration of these persisting abnormalities remains unknown and warrants further exploration.
One substantial limitation of this study was the time taken to complete the work set against the rapid increase in the number of studies being published. Most studies were from high income countries, limiting generalisability to countries with less developed healthcare systems. As with any evidence synthesis, confidence in the findings of the meta-analysis relies on the quality of the included studies, heterogeneity of study design and consistency of effects, and precision. Studies estimating prevalence are prone to selection and information bias127. Heterogeneity in diagnosis of COVID-19, classification of symptoms and the method and duration of follow up, as well as variability of estimates, made synthesis less secure. Risk of attribution bias in symptom reporting combined with low numbers of studies with control groups further limits the ability to draw firm conclusions. Assessment of publication bias was only possible in studies recruiting from hospital settings, (due to numbers required for meaningful analysis) and analysis showed potential small study effects in this group. Few studies reported results stratified by age and there was no data on differences in patterns or prevalence of symptoms in different ethnicities, both of which need consideration in developing age and culturally appropriate resources and services.
This review adds to the evidence that some CYP experience effects of COVID-19 that last longer than four-weeks, and describes the most common prolonged symptoms, risk of prolonged symptoms and broader sequalae of the acute infection. It also highlights gaps in the evidence. Further studies are now needed to better characterise this condition, to develop treatment plans for affected CYP and to plan appropriate services to support them. These should ideally recruit from community settings, include population-based control groups and use standardised definitions and outcome measures where possible.
Keele data repository: Supplementary data to Long-term outcomes of COVID-19 infection in children and young people: a systematic review and meta-analysis. https://doi.org/10.21252/6xgg-pv56128
This project contains the following underlying data:
Keele data repository: PRISMA checklist for Long-term outcomes of COVID-19 infection in children and young people: a systematic review and meta-analysis. https://doi.org/10.21252/6xgg-pv56128
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We wish to acknowledge our translators, Anthony Burton and Dahai Yu. A previous version of this article has been published as a preprint and can be accessed as https://doi.org/10.1101/2023.04.04.23288110.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)