Keywords
HIV, non-communicable diseases, sub-Saharan Africa, diabetes, hypertension, decentralization, community integrated care
In response to the growing burden of chronic diseases in sub-Saharan Africa, where innovative and cost-effective health solutions are imperative, this study outlines a protocol for a cluster-randomized trial that compares integrated community-based care with integrated facility-based care to improve access and outcomes for patients with HIV, diabetes, or hypertension.
We will conduct a pragmatic cluster-randomized trial comparing integrated community care with integrated facility care in Tanzania and Uganda. Patients living with HIV, diabetes, or hypertension, stable on treatment in health facilities, will be organised into groups of approximately 8–14 persons and randomly assigned to integrated community or facility-based care.
The study has two co-primary endpoints: a composite endpoint of glycemia and blood pressure control among individuals with diabetes and/or hypertension and suppression of plasma viral load among people living with HIV.
Participants will receive their drugs, adherence support, and monitoring at a community venue in the integrated community arm. Those randomised to the control arm will receive integrated facility-based care. All study participants will be followed up for 12 months.
A sample size of 116 groups will provide over 80% power to detect an absolute difference in blood pressure and blood glucose control of 10% at the 5% two-sided significance level. For HIV viral suppression, the trial will have over 80% power to show non-inferiority with a delta margin = 8.5%, 7.5%, and 5.5% assuming viral suppression is 85%, 90% and 95% respectively. To allow for loss to follow-up, our target for enrolment is 124 groups, each comprising an average of 14 participants.
An economic evaluation within the trial will be conducted to estimate the cost and cost-effectiveness of integrated community care compared with integrated facility care. This will be complemented by a built-in social science process evaluation.
Ethical approval was granted by the Research Ethics Committees of the University College London (UCL), the National Institute of Medical Research (Tanzania), and the Uganda Virus Research Institute (Uganda). The findings will be disseminated through journal publications and meetings with key stakeholders.
ISRCTN Registry: ISRCTN15319595, registration date: 07 June 2022.
This study aims to investigate better healthcare strategies for people with chronic diseases such as HIV, diabetes, or hypertension in sub-Saharan Africa. It seeks to determine whether community-based care offers a more innovative and cost-effective solution than traditional hospital or clinic care. The study will compare two types of care: one where all patients regardless of their diagnosis receive treatment and support in their community, and another where they go to medical facilities.
The patients who are stable on their treatments will be grouped and randomly assigned to either integrated community care or facility care. The primary objectives are to assess how well community care does in controlling blood sugar and blood pressure in diabetes and hypertension patients and in keeping the viral load down in HIV patients.
The research plans to track the health outcomes of these patients over 12 months as they receive integrated care. It aims to involve 124 groups of about 8–14 people each to ensure reliable results. The study will also evaluate how cost-effective community care is compared to facility care and will include an analysis of the social aspects involved in this type of care.
In simpler terms, this research is about testing whether taking care of people with certain chronic diseases in their communities is as effective and more affordable than caring for them in hospitals or clinics, with the hope of improving health access and outcomes in sub-Saharan Africa.
HIV, non-communicable diseases, sub-Saharan Africa, diabetes, hypertension, decentralization, community integrated care
In sub-Saharan Africa (SSA), approximately 5% of adults are estimated to be living with diabetes and 25% with hypertension1,2. Annual premature deaths from these conditions are estimated to be around two million3. Only a small proportion of individuals with these conditions are in regular care4,5 and among those who are, the control of blood pressure and glycemia is poor5–8.
In contrast, the coverage of HIV services offering antiretroviral therapy exceeds 80%, and the rates of HIV viral suppression are very high9. HIV services are accessible and increasingly available from both health facilities and communities10. However, health services for HIV and other conditions are provided in a vertical, disease-specific manner6. While this approach allows health services to provide dedicated care, it results in duplication of services11 and can be costly for patients with multiple chronic diseases who have to attend multiple clinics.
In collaboration with healthcare managers and policymakers, we recently evaluated a model of integrated care for patients with HIV, hypertension, and diabetes (the “one-stop clinic”) in primary care facilities in Tanzania and Uganda compared to vertical care for these conditions. Patients received services for these health conditions at the same location on the same appointment visit. Medical records, laboratory, and pharmacy services were also integrated. The findings showed that integration resulted in high retention in care for people with diabetes and hypertension and did not adversely affect viral suppression rates among those with HIV12. We also found that it was acceptable13–16, and compared with vertical care, integrated care for patients with multiple health conditions was cost-saving from the provider's perspective12. This is key for policymakers in SSA countries who need to decide whether to prioritise integrated care given limited government budgets for HIV and non-communicable conditions.
However, the challenge of health facility care, whether integrated or vertical, is meeting the demand with a severe shortage of qualified clinical health care workers. Patients often have to travel long distances and incur significant costs to attend clinics. More efficient ways of organizing care, such as decentralization to the community, are needed. Experience in HIV community care indicates that it is feasible and can bring additional benefits13–15.
Integrated community care has the potential to reduce congestion in health facilities. This would ensure that facility services are reserved for patients whose clinical conditions calls for more skilled care. By decentralising care to the community, patients can avoid travel and waiting times, save transportation costs, and reduce indirect costs associated with loss of productivity and income. Moreover, integrated community care can lead to better health outcomes due to more personalised care.
However, community care poses some unique challenges. Patients may face stigmatisation if their condition is disclosed, and community sites may not offer the quality or range of services available in health facilities. Healthcare in a community setting is typically provided at specific times, which means that patients may not have the flexibility to receive care at a time that is convenient for them, as they would in a healthcare facility.
Community care would involve a major change in health care delivery, and a rigorous evaluation is crucial. Here we describe the design of a large pragmatic cluster randomized trial to evaluate the effectiveness of integrated community-based management of HIV, diabetes, and hypertension compared to integrated care of these conditions in facilities.
Our group, RESPOND-AFRICA, has established a strong relationship with health services in Tanzania and Uganda through previous trials on integrated facility-based care. We have an active Patient and Public Involvement (PPI) group that engages with researchers throughout the entire study cycle. Together with the researchers, the PPI group conceptualized and defined the research questions and designed the INTECOMM study.
The PPI group consists of key community stakeholders, including policymakers, patients’ representatives, health service providers, such as health facility managers and front-line healthcare workers, NGOs providing care for HIV and NCDs, civil society organizations, such as the National NCDs Alliance, Network of People Living with HIV, the Tanzania Diabetic Association, and community advisory boards (CAB). Our aim is to leverage patient and public involvement to ensure that our research priorities, questions, and design are aligned with the insights, needs, and experiences of individuals living with chronic conditions.
In the INTECOMM trial, we seek to answer key questions about whether integrated care for HIV and NCDs could be decentralized to community settings as a response to key needs observed in facility settings. During stakeholder engagement meetings, issues such as clinic congestion, scarcity of human resources for health, and poor control of glycemia and blood pressure were discussed. As a result, stakeholders proposed and advocated for the conceptualization of decentralized care and discussed how it could be designed and evaluated. This trial is grounded in the practical realities of chronic disease management in low-resource settings with high disease burden in Africa.
The PPI group contributed significantly to the choice of primary and secondary outcome measures for this study. Their insights were instrumental in identifying key themes for exploration alongside traditional clinical measures, broadening the study’s scope to better evaluate the intervention. Patient representatives, healthcare workers, policymakers, and other stakeholders were involved in discussions about which patients would benefit most from community-integrated care (inclusion and exclusion criteria) and how they should be recruited into community care from their health facilities. The PPI group's input remains pivotal during the recruitment phase as they will receive updates and provide feedback during CAB and steering committee meetings.
Throughout the study, an iterative dialogue will be maintained with the PPI group, incorporating their feedback into the ongoing research cycle. Findings from the study will be shared with this group, and they will provide guidance on how best to communicate the main messages from the study to participants and the community. Through ongoing workshops and meetings, they will continually guide on contextually appropriate avenues for public dissemination.
The integrated community model was designed using lessons learnt from HIV community care. Healthcare systems, patient diagnosis and management tools, and implementation strategies developed to provide continuity of care for HIV in SSA can efficiently and effectively support patients with other chronic conditions such as diabetes and hypertension11.
To develop a community care model to be tested in this trial, a scoping review was conducted to identify existing or potential interventions for managing HIV, diabetes, and hypertension through integrated care (Supplemental material 1)17. The scoping review was based on a framework developed by Arksey & O’Malley and refined by Levac et al., which was further validated through a broad interprofessional team experience18–20. The framework consisted of five steps that included; identifying the research question, identifying relevant studies, selecting the studies, charting the data, and collating, summarising, and reporting the results.
Based on the findings of the scoping review, we categorised community-based care into six general design groups. These included improving access to medications (specifically ART), providing treatment support programmes, multifaceted support programmes, home visits from community health workers, support through SMS text messages, and differentiated care. Research comparing patient outcomes between community and alternative care found that outcomes improved or at least no worse than the standard of care, although the study design and the quality of implementation varied between different studies. This body of research suggests that a suite of interventions may be more effective than a single measure.
From a range of government policies from Tanzania & Uganda, we found four community-based interventions recommended for the treatment of patients with HIV. These interventions are accompanied by clear and detailed methods for their implementation in practice. Several tentative models of integrated community care were developed using the HIV community care models as a template. These were presented to policy makers, healthcare providers, and patient representatives in Uganda and Tanzania.
In these workshops, stakeholders, together with researchers, addressed important questions such as; "What are the challenges associated with the different proposed models of community integrated care and how can they be mitigated from the design stage?", "Where in the community should services be provided?", "Which patients should receive care in the community?", "How often should routine blood pressure and glycemia monitoring be performed?", "How often should patients visit the facility to see a doctor?" and "What kind of community model would be attractive to policymakers and what type of data would they need for policy considerations?". Through these deliberations, a model of integrated community care was developed for HIV, diabetes, and hypertension, which will be tested in this trial.
Community care is an innovative approach that will include major changes to the way health care is currently organized. For example, it will involve medicines being dispensed by non-pharmacists in a nonclinical setting, as well as monitoring the control of chronic conditions in the community. It is crucial that this approach is rigorously evaluated in a trial to inform policy in Tanzania and Uganda, as well as to facilitate generalization to other countries in Africa. Due to the need for rigorous evidence, we planned a pragmatic cluster randomized trial comparing community-based integrated care for HIV, diabetes, and hypertension with integrated services offered at health facilities in Tanzania and Uganda.
The trial will involve the recruitment of residents of the catchment areas of 10-16 health facilities in Tanzania and Uganda, which are mainly urban and peri-urban. In each facility, patients who meet the inclusion criteria will be formed into groups of approximately 8–14 people based on their address and their disease/condition. Grouping of study participants will be done collaboratively between the research team, the clinical team of the health facility, and lay workers who know the area.
Patients with HIV, diabetes, and/or hypertension who live close together in the same ward or parish and have met the eligibility criteria (Table 1) and provided informed consent will be placed in a trial group. Each group will have a 2:1 ratio of participants living with diabetes and/or hypertension to those with HIV. Patients will be recruited consecutively as they come in for their scheduled clinic visit and by reviewing patient records at the facility. A group is finalized when the target number and ratios are met. The group will then be randomized to either of the study arms (Figure 1 – The trial schema).
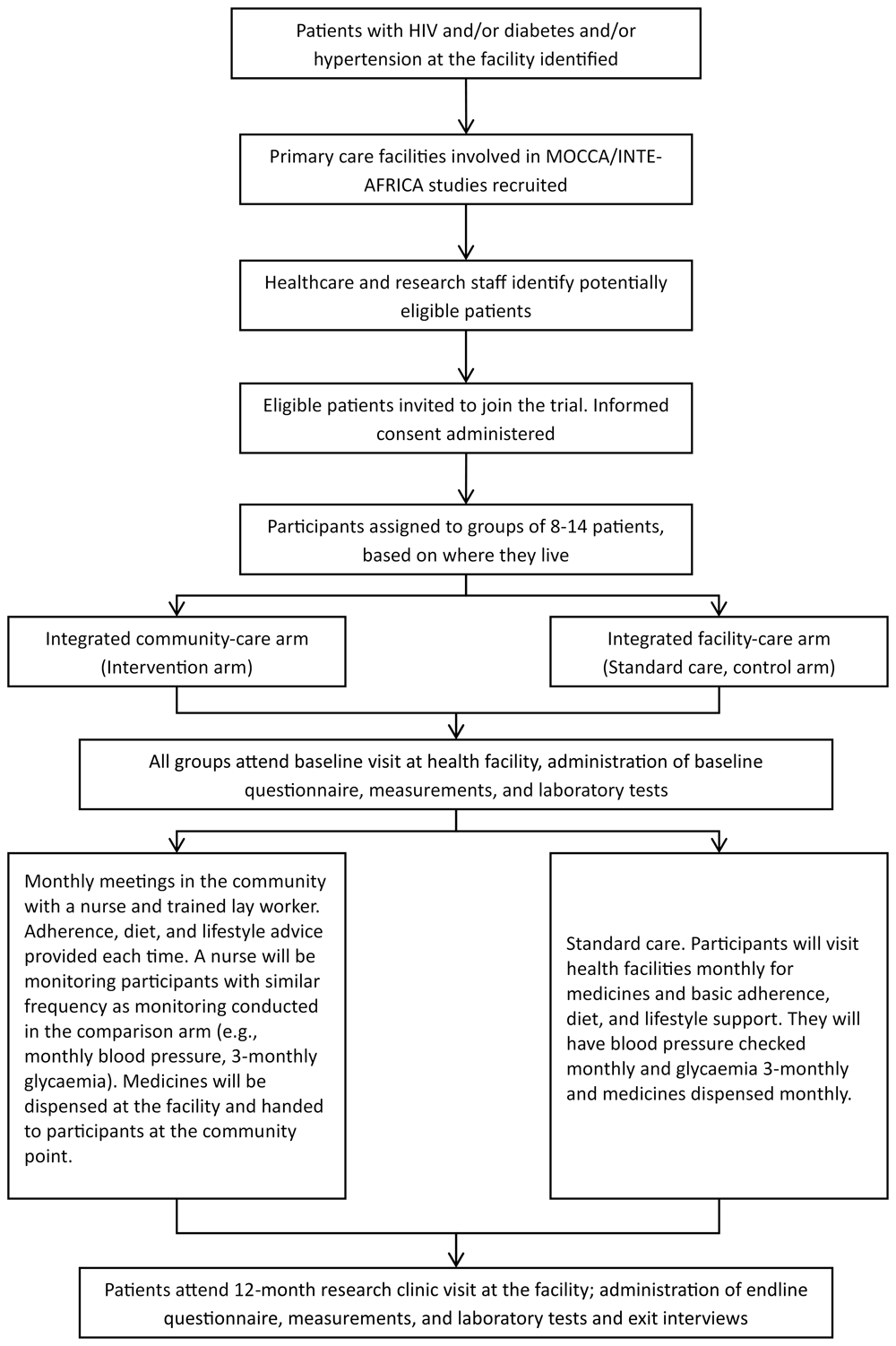
Abbreviation: MOCCA – Management of Chronic Conditions in Africa, INTE-AFRICA – Integrated management of HIV, diabetes and hypertension in sub-Saharan Africa.
Randomization will be computer-generated. To ensure allocation concealment, the randomization procedures will be built into the study’s electronic database. The group arm allocation will be visible only after randomisation. It will be stratified by health facility, based on its infrastructure. This allows the results of the findings to be generalizable. Equipoise is robust, as we are truly uncertain which of the trial arms is most likely to benefit the patient21. This trial protocol is reported using the SPIRIT Checklist for reporting protocols of randomised controlled trials22.
The Integrated Community care (intervention) arm. Groups of 8–14 patients randomized to community-based integrated care will hold their first group meeting at the health facility. In this meeting, a nurse and a trained lay worker (community health worker in Tanzania and village health worker in Uganda) will lead the group in choosing a group leader and suggest venues in the community where they would like to receive care. The nurse, the trained lay worker, and the group leader will then visit these venues and agree on the most suitable in terms of accessibility and comfort for a group meeting. They will then map the community stakeholders around the proposed venue and hold an engagement meeting to inform them about the study prior to the first meeting at the community venue.
The nurse and a trained lay worker will prepare patient files one day before the community group meeting, and a health facility clinician will write prescriptions for patients at community sites. The facility pharmacist will then dispense these medications ready for pickup by a nurse.
Group meetings will occur monthly. Patients will receive health education, monitoring of their blood pressure and blood sugar, and the nurse and lay worker will deliver drugs in the community. The nurse will also conduct a clinical review to assess the progress of the patients and refer those who have developed danger signs to the facility for further management by a physician. Adherence and behavioural information and support will be provided by a trained lay worker supervised by the nurse.
The baseline and end of the study (12th month) visit will occur at the health facility and monthly follow-up meetings will occur in the community. The trained lay worker will identify and trace patients who miss their community visit. They will be instructed to visit the health facility to pick their drugs and be informed of their next community meeting.
The integrated facility care (control) arm. Participants randomised to the control arm will receive integrated care in health facilities led by a clinician. They are effectively in artificial groups of 8–14 patients to allow for comparison.This study will be conducted in facilities in Tanzania and Uganda that were involved in previous studies on integrated facility-based care. In Uganda, some facilities continued the integrated clinics after the trial ended. However, facilities in Tanzania did not continue providing integrated care, as the decision to do so required evidence of effectiveness for implementation. Therefore, integrated clinics will be re-established in these facilities for the current trial.
Participants living with HIV, diabetes, and/or hypertension will receive care from a single clinic. They will have one registration and waiting area and will be managed by the same clinicians, nurses, counsellors, and other staff. There is one pharmacy where drug dispensing is integrated. Patient records will be integrated, and laboratory samples will be managed and tested in the same laboratory where possible.
Patients will receive health education and clinical monitoring, collect their drugs from the health facility pharmacy, and receive adherence and behavioural information and support as standard provided by the health facility to all patients.
The standard of care for participants who declined to join the study or withdrew. The current standard of care for HIV, diabetes, and hypertension varies depending on the level of the health facility. Although patients with diabetes or hypertension are typically seen in outpatient clinics in smaller hospitals, larger facilities have separate specialised clinics for these conditions.
HIV care features separate waiting areas, consultation rooms, and a dedicated pharmacy. Medical records and laboratory samples are managed separately from those of diabetes and hypertension. Patients with HIV have different schedules depending on their level of stability. They usually attend care three or six monthly for routine appointments when stable, while patients with diabetes and hypertension attend monthly or even more frequently. Patients with multimorbid chronic conditions must attend different clinics on different days since the clinics are run separately.
This trial will be run in close to real-life conditions, and participants will be managed by government staff in public facilities and routine staff in private or non-profit facilities. In both arms of the trial, we will train clinical staff on how to manage HIV, diabetes, and hypertension. They will also receive training on the trial protocol and standard operating procedures. The staff members will then undergo on-the-job training regularly. Clinical staff experts in one disease will support staff who are learning to manage the other two conditions in integrated care clinics. For example, doctors who have historically treated patients with diabetes and hypertension would periodically observe those in HIV clinics and provide constructive feedback and support.
Patients randomised to the control arm will receive care in the integrated clinic of the health facility and will be seen by routine staff at the facility. Those receiving care in the community will also have their community clinical review notes and prescriptions recorded in their hospital files by facility staff, while the facility pharmacist will dispense and package the drugs to be delivered to the community. This approach is economical, allows facility personnel to learn transferable clinical skills, and is sustainable beyond the life of the trial if the facility decides to carry out this mode of service delivery, as happened in Uganda23.
The trial has two coprimary endpoints, measured in 2 different populations:
1. Plasma viral load suppression for participants living with HIV infection are defined as having less than 1000 copies per ml (or reported as undetectable viral load)
Previous studies of integration of services at the facility level showed that integration of services did not adversely affect gains from vertical HIV programming, as viral suppression and retention in care were comparable in both arms. With the decentralization of integrated care to the community, it is important to evaluate how integrated community care will affect HIV viral suppression in the community compared to standard integrated care in the health facility. A plasma viral load of less than 1000 copies/ml is the WHO marker for viral suppression adopted by the country guidelines in Tanzania and Uganda24.
2. A composite of glycemia and blood pressure control (<7.0mmol/l and <140/90 mmHg) for those living with diabetes and hypertension, respectively.
Control of blood pressure and blood glucose is poor even when retention is high6. Good control of chronic conditions is important at the patient and health service level, as this will prevent further progression of the disease and the development of complications. The management of complications is costly, reduces quality of life, and leads to poor outcomes for patients11.
Community care offers a more personalized approach to patient management by taking patients out of clinical settings and placing them in smaller groups. Although some may perceive it as an inferior service, this approach allows health care staff to provide better services without the time pressure caused by the high volume of patients in the health facility. It is crucial to assess the impact of this change on the management of hypertension and diabetes, specifically the control of blood pressure and blood sugar, in patients with these conditions.
Secondary endpoints will include the equivalence of glycemia and blood pressure control, retention in care, costs, and cost-effectiveness.
Retention in care is fundamental to disease control. In our previous studies on integrated care in health facilities, patients maintained high retention. With the scepticism and controversy surrounding community care, it is important to assess whether participants will remain in care and how this might differ between participants with different conditions.
A within-trial cost-effectiveness analysis guided by the Gates reference case on cost-effectiveness will be conducted25. Cost analyses will estimate the total societal cost of delivery, including provider and patient or household costs. Provider cost data will be collected prospectively at both sites through project accounts, a facility-based cost capture tool, project records, and interviews with project staff. Data on patient costs will be collected using an endline questionnaire that asks about medication and other medical costs, travel costs, and time spent travelling and receiving care for a given visit. Any additional patient care-seeking in the month before the visit will also be captured. Costs will be captured in Ugandan and Tanzanian shillings. All costs will be adjusted for inflation using the Consumer Price Index (CPI) for Uganda and Tanzania and will be converted to 2023 International Dollars (INT$) using the 2023 Purchasing Power Parity (PPP) conversion factor for both countries26.
Results will be presented in terms of incremental cost-effectiveness ratios (ICERs), calculated as the arithmetic mean difference in cost divided by the arithmetic mean difference in effect between the community and facility arms. ICERs will be calculated for the co-primary outcome measures, i.e., plasma viral load suppression and a composite of glycemia and blood pressure control. ICERs will also be calculated based on the self-reported Luganda and Swahili versions of the EQ-5D-3L27, to generate an incremental cost per quality-adjusted life year gained. This will improve the comparability of the results with other studies and health interventions25. Sensitivity analyses will be conducted to assess the impact on cost-effectiveness results, of changes in parameters with the greatest uncertainty, or with the greatest impact on the total costs. Costs and outcomes will be converted to present values using an annual discount rate of 3% in the base-case, and annual rates of 0% and 6% in sensitivity analyses25. Reporting of economic evaluation results will be guided by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 202228. A separate economic evaluation protocol will describe planned analyses in more detail.
A process evaluation will be conducted alongside the intervention29. The aim of the process evaluation of this community-based integrated care trial is to explore implementation from diverse perspectives (patients, community health workers and volunteers, healthcare providers, policymakers, NGOs, community members and leaders) to gain insight into the influence of socio-structural and contextual factors on service integration at the community level.
Glycaemia and blood pressure control. We hypothesize that community care will be superior to facility care because health services and support will be more accessible as it is provided close to the patient’s home and more personalised due to the small size of the groups run by a nurse alongside a community/village health worker and peer leaders. In an earlier pilot study on integrated care (MOCCA study)6, which was conducted in 10 facilities with more than 2,000 participants followed for 6–12 months, 54% of hypertension participants had good control of blood pressure (BP<140/90 mmHg) and 39% of diabetes had achieved fasting glucose <7 mmol/L6.
We assume that 50% of participants in the control arm with diabetes or hypertension will achieve a good level of control after 12 months, that is, blood pressure <140/90 mmHg and fasting plasma glucose <7 mmol/L. This is plausible since we will be recruiting stable patients who are in regular care and should therefore start with a better level of control, and patients in both arms will be monitored and supported as per guidelines.
The calculation assumes 80% power and a 5% two-sided significance level. Table 2 shows the number of participants needed to compare integrated community care with integrated facility-based care for the control of blood pressure and/or glycemia.
Therefore, with 116 groups of 8 people each with diabetes or hypertension, the trial will have 80% power to detect a difference in the absolute rise of 10% (i.e., 50% versus 60% achieving good control in the 2 arms would be statistically significant at the 5% two-sided significance level if the intra-class coefficient is 0.02 or lower) (Table 2). In the MOCCA study (a pilot study on integrated facility based care in Tanzania and Uganda), the risk differences between baseline and the end of the study among participants with hypertension were 10.5% and among those with diabetes 7.4%, after excluding those in care for less than 6 months. Therefore, we will use an absolute increase of 10%, as power will be very high for differences larger than this.
HIV viral load suppression. For the HIV viral suppression endpoint, the MOCCA study showed viral suppression close to 90%, the UNAIDS target, in this population6. We hypothesize that patients in the community arm will be able to achieve and retain viral suppression similar to those attending care at the facility-integrated clinic. This is because they will have the same access to medication, monitoring, adherence support, and counselling. Thus, the primary aim is to show non-inferiority in the community care arm.
The non-inferiority margin in trials is often set at 10% (i.e., that the upper one-sided 95% CI of the difference between the control and intervention arm in terms of viral suppression will be within 10%). If we form 116 groups in total, each with 4 HIV participants, then the trial will have more than 80% power to show non-inferiority at delta= 8.5%, 7.5%, and 5.5% assuming viral suppression is 85%, 90% and 95%, respectively (assuming an intraclass coefficient of 0.02).
Therefore, we need a sample size of 116 groups, each comprising 12 persons, of which 8 should have diabetes or hypertension and 4 should have HIV. This equates to 1,392 evaluable participants in total. We propose enrolling 124 groups to allow for just over a 5% loss to follow-up in the number of groups. In each of these 124 groups, we will enrol 14 persons to allow for a loss to follow-up of just over 10% in the number of participants. Thus, our target sample size is 1,736 participants. Approximately half of the enrolment will be in Tanzania and half in Uganda. Thus, each country will enrol a total of 62 groups and 868 participants.
All members of the study team will be trained in good clinical practices (GCP), the study protocol, and standard operating procedures. Study group meetings will be conducted regularly to review the study's progress, address difficulties, and provide feedback on the implementation of the study. The trial will also be regularly monitored following the study monitoring plan. An electronic database custom-designed for the trial will be used that has a built-in data type and logic checks for real-time electronic data validation. Any data modifications are tracked on a comprehensive electronic audit trail and any changes to the source code are tracked and versioned. Data can be created, viewed, modified, deleted, or exported by delegated personnel according to their access roles associated with their personal accounts. Data security is ensured by using authentication and encryption to make subject identity and personal health information unreadable and undecipherable to unauthorised individuals. The study data are not stored on any device in the field.
An independent Data Safety Monitoring Board (DSMB) will be responsible for evaluating outcomes and receive and review reports of serious adverse events.
The statistical analysis will employ Generalised Estimating Equations to account for clustering based on the intention-to-treat principle. The primary analysis will compare the proportions of patients who achieve viral suppression, blood pressure, and glycaemic control.
Cost data analysis will calculate the costs of healthcare delivery and the societal costs incurred by patients to access healthcare. These data will then be linked to effectiveness estimates to determine potential cost-effectiveness.
Qualitative data analysis will be conducted thematically using NVivo, an electronic data management package. This method will facilitate the identification of key themes and patterns in the data.
This study will be conducted in accordance with the Revised Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects) and with the laws and regulations of Tanzania and Uganda, as well as the Good Clinical Practices Guidelines.
The study protocol, information sheets and consent forms for patients were submitted for approval to the local National Ethics Committees for Health Research, and to the sponsor institution’s Research Ethics Committee at UCL.
Written approval from all Ethics Committees has been obtained; the National Institute of Medical Research in Tanzania (NIMR/HQ/R.8a/Vol. IX/3977 (28th April 2022)), the Uganda Virus Research Institute(GC/127/872 (23rd March 2022)), the Uganda National Council for Science and Technology (HS2278TS (13th July 2022)), the London School of Hygiene and Tropical Medicine Ethics Committee (28122 (31st August 2022)) and the University College of London (2382/001 (25th October 2022)), which is also the trial sponsor.
All research staff have undertaken Good Clinical Practice Training, and tailored, study-specific research training addressing ethical concerns and considerations for engaging participants in research study in each setting. As required, any changes to the protocol will be submitted to the approving ethics committee as an amendment.
Before data collection, the delegated research staff will read and explain the information sheet to the participants in Swahili or Luganda and answer any questions that may arise. The participant will be informed about the purpose of the study, the study procedures, and the risks and benefits of participation. The information sheet will also emphasise that participation in the study is entirely voluntary and that participants are free to withdraw at any time without compromising their standard care received at the study site.
If the individual agrees to participate, the participant and the research staff will write their full name, and date, and sign the consent form. In case of illiteracy, oral consent in the presence of a witness who is not part of the health personnel at the trial site is acceptable. In this case, the signature of the witness will be collected. The information sheet and a copy of the signed informed consent form will be given to the participant. The dates of the information and the signature of the consent form will be recorded in the electronic CRF database with the name of the research staff who obtained the informed consent.
The findings of the study will be disseminated through stakeholder meetings, local and international conferences, policy briefs, peer-reviewed journal articles, and publications.
This protocol is for a cluster-randomised trial that compares an integrated community care intervention with integrated facility care. It is large and simple and designed to generate evidence to inform policy considerations for the integration of HIV and NCDs. It is a multicountry multicentre trial and hence allows for greater generalisability of findings. Another strength of the trial is that it has been co-designed with community stakeholders; therefore, it addresses the real challenges facing patients in accessing treatment. This approach will facilitate implementation and a sense of ownership, ensuring that policymakers, patients, and other stakeholders trust and use the findings for advocacy and policy reform. The study also measures objective markers of effectiveness and involves a multidisciplinary team of experts.
A weakness of this trial is that it is impossible to blind participants and service providers, who may have a bias on which intervention is effective. To mitigate this, staff will be regularly trained on the importance of equipoise in trials and the trial endpoints are kept objective. Another weakness is that some clusters that could benefit from the trial may be excluded because they do not meet the numbers and ratios required, hence limiting the generalisability of the findings. This was accounted for by the large sample size of this trial involving 10–16 facilities across the two countries.
One of the main threats to the trial is the provision of suboptimal care in community settings by lay workers, which can compromise the safety and health outcomes of patients with chronic diseases. To address this problem, lay workers will receive comprehensive training before starting the trial, which will cover general concepts on hypertension, type 2 diabetes, and HIV, study protocol training, communication skills, and research ethics, and will receive refresher training frequently and continuous hands-on training from the nurse. In addition, a nurse will work closely with the trained lay worker to provide care to participants at community sites. The nurse will perform a clinical review of the patient that includes checking for any danger signs, performing a physical examination, reviewing the patient’s vitals (blood pressure and temperature when indicated), and fasting blood glucose results taken the same day in the community. Based on the clinical review, the nurse will decide to refer the patient back to the health facility or continue care at the community site. To avoid any potential oversight, the nurse and the lay worker will receive a comprehensive checklist of warning signs and symptoms. This checklist will serve as a prompt for an instant referral to the facility for further evaluation and treatment by a physician if a patient exhibits any of the aforementioned signs or symptoms.
Another threat is the lack of proper coordination and linkage with other services available at the health facility, such as viral load tests, routine blood tests, family planning, tuberculosis screening, and vaccines such as the COVID-19 vaccine. To mitigate this, patients in the community arm who are due for their routine viral load test and other blood tests, such as renal and liver function tests, will have the opportunity to receive care at the facility during the respective month. This way, they can access any additional care they need that is not available in the community. The study nurse will record each time a patient randomized to the community arm accesses care at the facility and the reason for their attendance.
It is also possible that community care might prove challenging for some participants. Community care will be available only once a month, and if participants miss that day, they will need to go to the health facility for their care. This could be particularly challenging during the rainy season. In the control arm, integrated care clinis and standard vertical clinics are available most days, so missing appointments will not be a major concern. If community care was available more frequently, as it would be in real life if it were scalled up, missing appointments would not be an issue for patients. Therefore, the trial setting tends to favour facility based care and in a real life setting, community care may work better than in a trial setting.
Meeting in small groups within the community could lead to inadvertent disclosure of the patient's status, as community care lacks the potential veil of anonymity that facility-based care affords patients. This could make it easier for patients to be identified as having a chronic disease, leading to stigma. To minimise the likelihood that this happens, drugs will be pre-packaged and marked with patient identifiers at the facility. Moreover, the nurse will provide one-on-one clinical review and drug delivery to each patient in a private space at the community venue to ensure confidentiality.
Furthermore, NCD drugs are often out of stock in public health facilities in Uganda and Tanzania, affecting patient adherence to treatment. In Uganda, patients pit resources by making a modest monthly monetary contribution to patient clubs, which can provide drugs to members at a subsidized price. In Tanzania, some patients receive their drugs through their health insurance scheme while others pay out-of-pocket. Economically deprived patients can receive government waivers, but stock shortages remain common. To address these challenges, a buffer stock of drugs will be donated to health facilities to be used when the stock runs out.
The trial has a high power to compare community with facility care aggregated across both countries. However, a concern is that implementation may differ between the countries due to the differences in health systems, governance, and the supply of medicines and diagnostics. If this happens, the analyses stratified by country will be vital, but this will have much less statistical power than the analyses aggregated across both countries.
In conclusion, the INTE-COMM study will test a pioneering approach of delivering integrated care for diabetes, hypertension, and HIV at the community level. If proven effective and economical, it could be scaled up, thereby providing greater access to evidence-based care for patients with diabetes and hypertension. This trial has the potential to generate groundbreaking insights and impetus for the expansion of integrated community care for HIV, diabetes, hypertension, and other NCDs and could significantly improve the way these conditions are managed in low- and middle-income settings.
Figshare: Community-based interventions for the integrated treatment of HIV and Diabetes and/or hypertension in Sub-Saharan Africa: Scoping review summary report. Preprint. https://doi.org/10.6084/m9.figshare.2572700717
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Figshare: SPIRIT checklist for ‘Integrated community-based management of HIV, diabetes, and hypertension in Tanzania and Uganda: protocol for a cluster-randomized trial’. https://doi.org/10.6084/m9.figshare.2572658422
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank all members of our PPI group who took part in workshops and discussions during the conceptualisation and designing of this study. Their dedication and insights underscored the indispensable role of meaningful community engagement in shaping impactful interventions.
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Primary Health Care
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 26 Jun 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)