Keywords
Invasive ventilation, Innovative ventilation strategies, Intensive care unit, scoping review
There is widespread interest in the use of innovative ventilation technologies to improve clinical outcomes across the 13–20 million people each year globally that receive invasive ventilation on an intensive care unit. This scoping review aims to summarise the volume and nature of evidence underpinning the use of 22 innovative ventilation technologies in adults and children.
We searched MEDLINE, EMBASE, Cochrane library and other key databases from 2010 to May 2024 for primary studies and systematic reviews that evaluated the use of 22 innovative ventilation technologies in adults and children requiring, or at risk of requiring, invasive ventilation. We defined an innovative ventilation technology as a ventilation approach not currently recommended by clinical guidelines due to lack of or uncertainty of evidence. We summarise findings as evidence maps.
Our search identified 22,274 records of which we included 851 studies (564 primary studies; 277 systematic reviews; 10 economic evaluation studies). Over 50% of studies focussed on non-invasive respiratory support strategies to reduce the risk of a primary tracheal intubation (n=319, 37%) or re-intubation (n=130, 15%). We identified ten or fewer studies for seven technologies, including phrenic nerve stimulation, artificial intelligence, and ultra-low tidal volume ventilation. Few studies include children (n=128, 15%) or report patient-focussed outcomes (n=19, 2%).
For many technologies despite being used in clinical practice, the available evidence is currently inadequate to determine its clinical effectiveness, particularly in children. Key technologies need to be evaluated in high-quality multi-centre clinical trials that report patient-focussed outcomes.
Each year in the UK, over 60,000 adults and 10,000 children require treatment from a breathing machine (ventilator) on an intensive care unit. This involves placing a tube into the windpipe and using a machine to support breathing. Going on a ventilator can be life-saving, but it can have significant side-effects. Many new methods and devices have been developed that aim to improve how we care for patients on ventilators, or to reduce the need for using a ventilator. In this project, we identify and describe published studies on 22 new methods or devices intended to help people on a ventilator, or at risk of needing a ventilator.
We searched several databases from 2010 to May 2024. We focused on 22 new methods and devices not currently recommended by clinical guidelines because people are unsure if they work. We summarise findings using tables and graphs, known as evidence maps.
We found 851 studies. These include 564 primary studies which provide new data; 277 systematic reviews which evaluate and summarise previous studies; and 10 economic evaluations which examine value for money. Over half of the included studies focus on methods and devices to reduce the need for a ventilator, n=319, 37%) or re-introducing a ventilator after patients had come off it, n=130, 15%). We identified ten or fewer studies for seven of the methods or devices. Few studies include children (n=128, 15%) or report patient-focussed outcomes (n=19, 2%).
There is a lack of evidence to determine whether many of the new methods or devices work particularly in children. High-quality clinical trials undertaken in different places and reporting outcomes important for patients are needed.
Invasive ventilation, Innovative ventilation strategies, Intensive care unit, scoping review
Each year, an estimated 13–20 million adults across the world receive invasive mechanical ventilation on an intensive care unit1. Invasive mechanical ventilation is a life-saving intervention, but it comes at substantial individual and societal cost. Around 30% of adults and 5% of children that require invasive mechanical ventilation die2–4. Survivors often report significant and sustained negative long-term effects on their health-related quality of life5–7. From a societal perspective, the financial cost of invasive ventilation involving an intensive care unit (ICU) stay is high8.
The desire to optimise outcomes in patients requiring, or at risk of requiring, invasive mechanical ventilation has driven ongoing developments in innovative strategies. There is tension between early implementation of technologies underpinned by sound physiological principles with evidence of improvements in surrogate outcomes, such as oxygenation, and the need to produce robust evidence of clinical and cost-effectiveness prior to implementation9,10. Previous research has shown that while some interventions are clinically effective, such as smaller tidal volumes11 and prone positioning during invasive mechanical ventilation12, other interventions may be ineffective or potentially harmful, such as high-frequency oscillatory ventilation in adults13,14.
On this basis, we identified the need to undertake a scoping review to inform clinical practice and research funding priorities by summarising the volume and type of evidence across a range of innovative ventilation technologies. We defined an innovative ventilation technology as an approach to ventilation that is not recommended by current clinical guidelines due to uncertainty of evidence or not yet addressed due to its novelty.
We conducted our scoping review, in line with a study protocol prospectively registered with the Open Science Framework (https://osf.io/szkqn/). Our review methodology was informed by the Joanna Briggs Institute methodological guidance for the conduct of scoping reviews15. The review is reported in line with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR)16.
This review is part of a larger programme of work which aims to map current evidence underpinning innovative ventilation technologies across adults and children, and to appraise evidence on clinical and cost-effectiveness of promising technologies prioritised by key stakeholders.
We convened an expert reference group to inform the project design, delivery, and dissemination. The expert reference group included service commissioners (represented through NHS England), clinicians, and patients and carers with personal experience of invasive ventilation. We invited professional organisations (e.g., Intensive Care Society, Paediatric Intensive Care Society) to nominate clinician representatives. We identified and invited patient contributors through our local patient and public involvement research group, which helped design this study, and our other existing networks, such as the NIHR Applied Research Collaboration West Midlands PPI Network. The group met to agree terms of reference of the group and support the study team in identifying and mapping a list of innovative technologies to be included in our scoping review. To support the process of classifying interventions, we presented and discussed our early ideas on how best to characterise the ventilation care pathway using a conceptual model/ taxonomy. The project PPI co-applicant (MT) worked closely with the research team to provide day-to-day project support, ensuring that the project remains focussed on the interests of patients and members of the public.
Our initial list of innovative ventilation technologies was developed through collaborator group knowledge and review of key clinical guidelines focussed on the care of invasively ventilated adults and children, including those developed by the European Society of Intensive Care Medicine, American Thoracic Society, Society of Critical Care Medicine and the UK Intensive Care Society17–24. We worked in partnership with an expert advisory group comprised of clinicians, patient representatives, researchers, and service commissioners with expertise across adult and paediatric intensive care to refine our list of technologies and identify additional technologies. Our final list comprised 22 innovative ventilation technologies (Table 1).
We identified our review framework as:
Population: intensive care and acute hospital patients requiring invasive ventilation or at risk of requiring invasive ventilation.
Concept: innovative ventilation strategy, defined as an approach to ventilation that is not recommended by current clinical guidelines due to uncertainty of evidence or not yet addressed due to its novelty. It can cover ventilation techniques, patient positioning concepts, and digital technologies to optimise ventilation. Examples include: airway pressure release ventilation, ultra-low tidal volume ventilation, closed-loop ventilation (e.g. adaptive support ventilation, neurally adjusted ventilatory assist), and digital technologies (e.g. artificial intelligence).
Context: evidence related to clinical effectiveness and cost-effectiveness potentially applicable to UK settings.
We searched MEDLINE (Ovid), Embase (Ovid), Science Citation Index and Conference Proceedings – Science (WoS), Cochrane Library (Wiley), CEA Registry and ACM Digital Library in October 2023 and ran update searches in May 2024 using a combination of free-text and MeSH terms. We limited our search to studies published since 2010 to avoid including historical interventions which proved either ineffective or impractical to implement in practice. No language restrictions were applied. Our search strategy was iteratively developed by an information specialist (RC), in collaboration with clinical experts.
For emerging technologies and technologies under-represented in the included studies, we searched the following grey literature sources: internet (Google) searches, ProQuest Dissertations & Theses Global database, proceedings of selected conferences of interest (published 2021 onwards) and websites of relevant organisations. Our detailed search strategy is available in Appendix 1 (Extended data).
We included studies that described the use of an innovative ventilation technology in adults and children at risk of requiring, or currently receiving, invasive mechanical ventilation. Our comparator of interest was standard care or another innovative ventilation technology. For innovative ventilation technologies used in patients receiving invasive mechanical ventilation, we included only intensive care unit studies. For innovative ventilation technologies used in patients at risk of requiring invasive mechanical ventilation, we included studies undertaken either on the intensive care unit or in the wider acute hospital. To be eligible, studies were required to report at least one outcome of interest, namely a patient-reported outcomes (e.g. quality of life), a clinical effectiveness outcome (e.g. mortality), a cost-effectiveness outcome, or a surrogate outcome that provides information about intervention efficacy (e.g. oxygenation).
Eligible study designs were randomised controlled trials, non-randomised comparative studies with a control group, systematic reviews, and economic evaluations. We excluded non-randomised studies with a repeated measure design as these were deemed to not include a comparator group. We excluded studies of neonates, defined as pre-term birth and/or those cared for exclusively in neonatal intensive care units. We excluded animal studies as, whilst they can be helpful in identifying therapeutic targets for clinical testing, the generalisability of findings from animal models of invasive mechanical ventilation to humans is limited25. A detailed overview of study eligibility criteria is available in Appendix 2.
Titles and abstracts were screened by a single review team member. For each review team member, a 10% sample of records was independently screened by another reviewer to ensure consistency in decision-making. An agreement level of 90% was deemed to represent an adequate level of agreement. Following title and abstract screening, two reviewers independently screened the full texts of potentially eligible studies, and recorded reasons for exclusion of ineligible studies. We resolved any disagreement through discussion or, if required, consultation with a third reviewer.
Eligible studies were coded/charted based on key features, namely: innovative ventilation technology type, patient population, stage of ventilation care pathway, study design, sample size, outcome measures, setting, and sources of funding. To support our mapping, we developed a taxonomy to define stage in the ventilation care pathway, based on previous studies26. Our taxonomy, as shown in Figure 1, comprised four stages, namely: innovative technologies to reduce the risk of requiring invasive mechanical ventilation (stage one); innovative technologies used during invasive mechanical ventilation to support treatment of acute respiratory failure, including optimisation of oxygenation, and to minimize ventilator induced lung injury (stage two); innovative technologies to support the process of weaning from invasive mechanical ventilation (stage three); and innovative technologies to reduce risk of re-intubation following extubation (stage four).
An individual reviewer extracted data from each study. The extraction in 20% of studies was checked by a second reviewer and disagreements resolved by discussion. No quality assessment was undertaken for included studies, in line with commonly adopted approaches to undertaking scoping reviews16.
We synthesised and summarised data in two complementary ways.
Narrative summaries: We presented a series of summary tables to show the volume and nature of evidence for each innovative ventilation strategy across all patients and for key sub-groups (e.g. population type (adults/ children), and stage of the invasive ventilation care pathway). These were accompanied by brief texts highlighting key features of the evidence base.
Evidence maps: These provide visual representation of all evidence across all patients and innovative technologies27. The evidence maps were created in two formats: first we created a Sankey diagram using the online SankeyMATIC to illustrate the complex relationships between key features of included studies; second we produced detailed cross-tabulation of the number of studies for individual innovative ventilation strategies broken down by key study features, separately for adults and children.
Our search identified a total of 22,274 records after de‐duplication and removal of some publication types. After screening of titles and abstracts and removal of 20,937 irrelevant records, we retrieved 1337 potentially relevant reports for detailed assessment. After assessment of the full‐text papers, we included 851 studies. A summary of the search results and study selection process is displayed in the study selection flow diagram (Figure 2). A list of all the included studies is provided in Appendix 3.
The included studies were mapped onto 22 innovative ventilation strategies (Table 2). Most studies focused on the use of non-invasive respiratory support strategies to either prevent the initial need for invasive mechanical ventilation (n=319, 37%) or prevent the need for re-intubation following tracheal extubation (n=130, 15%). Other areas with a high number of studies included closed-loop ventilation modes (n=78, 9%), extracorporeal membrane oxygenation for acute hypoxaemic respiratory failure (n=79, 9%), neurally-adjusted ventilatory assist (n=63, 7%) and airway pressure release ventilation (n=36, 4%). Technologies where few eligible studies were identified included driving pressure/ mechanical pressure limited ventilation (n=13, 1%), mechanical insufflation-exsufflation (n=12, 1%), phrenic nerve stimulation (n=2, <1%) and lung and diaphragm protective ventilation (n=1, <1%). Figure 3 illustrates the volume and key features of evidence across the four stages of the ventilation care pathway.
| Studies (n=851)1 | Adults only (n=706) | Children only (n=128) | |
|---|---|---|---|
| Innovative ventilation technology- n(%)† | |||
| Non-invasive respiratory support to prevent tracheal intubation | 319 (37%) | 251 (35%) | 65 (51%) |
| Non-invasive respiratory support to prevent re-intubation | 130 (15%) | 113 (16%) | 14 (11%) |
| Extracorporeal membrane oxygenation in AHRF | 79 (9%) | 72 (10%) | 5 (4%) |
| Extracorporeal carbon dioxide removal | 22 (3%) | 20 (3%) | 0 |
| Phrenic nerve stimulation | 2 (<1%) | 2 (<1%) | 0 |
| Lung and diaphragm protective ventilation | 1 (<1%) | 1 (<1%) | 0 |
| Sustained inflation recruitment | 32 (4%) | 29 (4%) | 3 (2%) |
| Brief recruitment | 32 (4%) | 30 (5%) | 2 (2%) |
| Closed loop ventilation | 78 (9%) | 71 (10%) | 2 (2%) |
| Automated spontaneous breathing trial | 11 (1%) | 10 (2%) | 1 (1%) |
| Driving pressure/ mechanical pressure limited ventilation | 13 (1%) | 12 (2%) | 0 |
| Ultra-low tidal volume ventilation | 9 (1%) | 8 (1%) | 1 (1%) |
| NAVA | 63 (7%) | 43 (6%) | 17 (13%) |
| Passy Muir valve | 1(<1%) | 1(<1%) | 0 |
| Cuff deflation | 1(<1%) | 1(<1%) | 0 |
| Awake prone positioning (non-COVID-19) | 0 | 0 | 0 |
| Artificial intelligence | 3 (<1%) | 1(<1%) | 0 |
| Airway pressure release ventilation | 36 (4%) | 34 (5%) | 1 (<1%) |
| High-frequency oscillatory ventilation (children) | 24 (3%) | N/A | 21 (16%) |
| PEEP optimisation strategies | 29 (3%) | 28 (4%) | 0 |
| Mechanical insufflation-exsufflation | 12 (1%) | 11 (1%) | 0 |
| Personalised strategies (including phenotypes) | 5 (<1%) | 5 (<1%) | 0 |
| Study type/ sample size | |||
| Systematic reviews | |||
| Number- n (%) | 277 (33%) | 232 (33%) | 36 (28%) |
| Number of included studies- median (range) | 10 (1 to 168) | 9 (1 to 168) | 6 (1 to 54) |
| Randomised controlled trials | |||
| Number- n (%) | 327 (38%) | 283 (40%) | 49 (38%) |
| Number of participants- median (range) | 71 (6 to 2427) | 80 (10 to 1013) | 40 (6 to 2427) |
| Observational studies | |||
| Number- n (%) | 237 (28%) | 183 (26%) | 43 (33%) |
| Number of participants- median (range) | 90 (9 to 53654) | 101 (9 to 53654) | 137 (30 to 17643) |
| Economic evaluations | |||
| Number- n (%) | 10 (1%) | 10 (1%) | 0 |
| Ventilation care pathway stage- n(%)† | |||
| Stage one | 343 (40%) | 286 (40%) | 76 (59%) |
| Stage two | 289 (34%) | 264 (37%) | 34 (26%) |
| Stage three | 161 (19%) | 123 (17%) | 12 (9%) |
| Stage four | 147 (17%) | 141 (20%) | 18 (14%) |
| Participant age- n(%) | |||
| Adult | 706 (83%) | ||
| Children | 128 (15%) | ||
| Adults and Children | 17 (2%) | ||
| COVID status of participants | |||
| COVID-19 | 99 (12%) | 96 (11%) | 0 |
| Not COVID-19 | 726 (85%) | 587 (83%) | 128 (100%) |
| COVID-19 and not COVID-19 | 26 (3%) | 25 (3%) | 0 |
| Types of outcomes reported- n(%)† | |||
| Clinical | 787 (92%) | 645 (91%) | 120 (94%) |
| Surrogate | 517 (61%) | 501 (71%) | 76 (59%) |
| Patient-focussed | 19 (2%) | 17 (2%) | 0 |
| Health economic | 28 (3%) | 25 (3%) | 3 (2%) |
| Setting (number of centres)- n(%)‡ | |||
| Single-centre | 405 (71%) | 326 (69%) | 70 (76%) |
| Multi-centre | 166 (29%) | 148 (31%) | 22 (24%) |
| Setting (Continent) n(%)‡ | |||
| Europe | 206 (36%) | 178 (37%) | 26 (28%) |
| North America | 90 (16%) | 64 (13%) | 26 (28%) |
| South America | 29 (5%) | 24 (5%) | 4 (4%) |
| Asia | 198 (34%) | 165 (35%) | 28 (30%) |
| Australasia | 9 (2%) | 6 (1%) | 3 (3%) |
| Africa | 23 (4%) | 20 (4%) | 3 (3%) |
| Multiple continents | 18 (3%) | 17 (4%) | 1(1%) |
| Funding type- n(%)† | |||
| Commercial | 25 (3%) | 22 (3%) | 1 (<1%) |
| Non-commercial | 365 (43%) | 305 (43%) | 58 (45%) |
| No funding/not reported | 461 (54%) | 376 (53%) | 69 (54%) |
| AHRF, acute hypoxaemic respiratory failure; NAVA, neurally-adjusted ventilatory assist; PEEP, positive end-expiratory pressure | |||
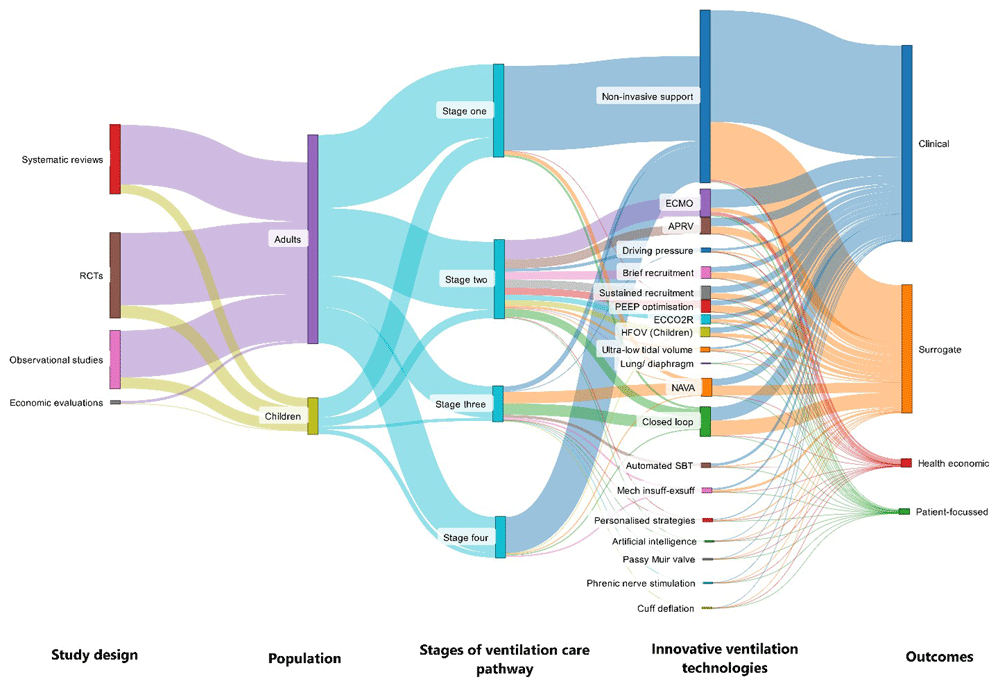
ECMO- Extracorporeal membrane oxygenation; APRV- Airway pressure release ventilation; ECCO2R- Extracorporeal carbon dioxide removal; HFOV- high-frequency oscillatory ventilation; NAVA- neurally adjusted ventilatory assist; SBT- spontaneous breathing trial
Geographically, primary research studies (n=564) spanned six continents, with 206 (36%) based in Europe, 198 (34%) in Asia, 90 (16%) in North America and 79 (14%) in other geographical areas. There were few studies that recruited across multiple continents (n=18, 3%). Figure 4 highlights the distribution of primary studies across countries.
Most of the included studies assessed outcomes of innovative ventilation technologies on adult patients (n=706, 83%) while only 15% of studies were conducted on children (n=128). The remaining studies (n=17, 2%) covered both adults and children.
The included studies evaluated technologies spanning across the four stages of the ventilation care pathway. Most of the included studies (n=343, 40%) evaluated the effectiveness of innovative technologies in reducing the risk of needing invasive ventilation. Many studies (n=289, 34%) assessed innovative technologies to support treatment of acute respiratory failure and minimise ventilator induced lung injury. A relatively smaller number of studies targeted interventions aimed at supporting the process of weaning from invasive ventilation (n=161, 19%) or reducing the risk of re-intubation (n=147, 17%).
We identified a large number of systematic reviews (n=277, 33%), with most of these reviews evaluating the effectiveness of non-invasive respiratory support strategies to either prevent the initial need for tracheal intubation (n=125, 45%) or prevent the need for re-intubation following tracheal extubation (n=40, 14%). Our scoping review included 564 (66%) primary studies; of which 327 (58%) were randomised controlled trials and 237 (42%) were observational studies. We identified only ten (1%) economic evaluations. The review identified more single-centre primary studies (405, 71%) than multi-centre studies (166, 29%). Industry funding was reported in 3% of the included studies (n=25), while 43% (n=365) were non-commercially funded. However, many studies (461, 54%) reported no receipt of external funding or provided no information about funding.
Most of the included studies used clinical (e.g., mortality and intubation rate, n=787, 92%) and surrogate outcomes (e.g., oxygenation parameters, n=517, 61%) to measure the effectiveness of innovative ventilation strategies. A limited number of studies reported the use of patient-reported outcomes (n=19, 2%) and cost-effectiveness outcomes (n=28, 3%).
Table 2 provides a breakdown of evidence by adults and children, and a detailed overview of evidence for each technology in adults and children is included in Appendix 4 and 5 (Extended data). For children, we identified 11 technologies where there was no primary research study. We also identified no economic evaluations or any study of COVID-19 respiratory failure. In general, the proportion of studies by funding type, reported outcomes and study type were similar across studies in adults and children. However, there tended to be more single-centre studies and North American studies in children. Sankey diagrams depicting the volume and type of evidence in adults and children can be found in Appendix 6 and 7 (Extended data).
Our scoping review, which charted data from 851 studies across 22 innovative ventilation technologies, found that most studies examined the use of non-invasive ventilation (n=449, 53%), included only adult patients (n=706, 83%), were a single-centre design (n=405, 71%), and reported clinical outcomes (n=787, 92%). We identified over 50 studies for three other technologies, namely extracorporeal membrane oxygenation for acute hypoxaemic respiratory failure (n-79, 9%), closed-loop ventilation (n=78, 9%), and neurally adjusted ventilatory assist (n=63, 7%). We identified fewer than 10 studies for eight technologies, including ultra-low tidal volume ventilation (n=9), phrenic nerve stimulation (n=2) and lung and diaphragm protective ventilation (n=1). Only 19 (2%) studies reported a patient-focused outcome, such as health-related quality of life.
Our finding of the infrequent reporting of patient-reported outcomes may, in part, reflect the relative novelty of some of the identified technologies. The choice of study outcomes is often a careful balance between practicality and value of information. In our review, 61% reported surrogate outcomes, such as oxygenation and ventilatory pressures. These outcomes may provide important early evidence of efficacy, but this early evidence of efficacy may not translate into clinical effectiveness. For example, early evidence on high-frequency oscillatory ventilation in adults showed it improved oxygenation28, but subsequent large randomised controlled trials reported that it likely provided no clinical benefit, and might be harmful14,29. Core outcome sets describe outcomes that are important to key stakeholders, thereby supporting the standardisation of outcome reporting across trials. The core outcome set for adult ventilation studies, developed in 201930, describes six core outcomes, including health-related quality of life. Our finding that few studies reported a patient-focused outcome, such as the health-related quality of life, may reflect the relative newness of the core outcome set.
We found that most primary studies were single-centre. Single-centre studies are often the first step in providing preliminary evidence of efficacy required to underpin future multi-centre randomised controlled trials. There is a need for caution in interpreting the finding of single-centre studies31. A recent systematic review explored the reproducibility of findings from single-centre to multi-centre intensive care randomised controlled trials32. Across sixteen single-centre randomised controlled trials that reported a significant effect on mortality, this effect was observed in a follow-on multi-centre trial on only one occasion.
One-third of the 851 studies included in this scoping review were systematic reviews. For some technologies, such as non-invasive respiratory support strategies, we identified more systematic reviews than randomised controlled trials. There may be important differences between reviews in population, type of non-invasive respiratory support and review methodology. However, there is an important concern that some reviews may have added little new information to the literature, creating a risk of redundancy and research waste. The potential harm and research waste that result from redundant systematic reviews have long been highlighted33 and measures for preventing their occurrence have recently been proposed34. Researchers should carefully reflect on the likely added value of any planned review to minimise the risk of research waste.
Our scoping review highlights the lack of high-quality evidence for an important number of technologies. This was particularly notable in children, where clinical practice and guidelines are frequently based on expert consensus and attempts to extrapolate indirect evidence from adults22,24. The challenge for the intensive care research community is how best to address this urgent need for further research and how to prioritise these technologies for future research. Intensive care research prioritisation exercises identify research on invasive mechanical ventilation strategies as a key priority for research35. Platform trials may provide an efficient strategy to simultaneously test multiple innovative ventilation technologies36. There may also be opportunities to streamline the delivery of standalone randomised controlled trials through collaborative working, such as the standardisation of data collection across trials.
Our scoping review has some limitations. First, whilst we iteratively developed a broad a search strategy across a range of databases to identify studies across a range of technologies, it is possible that our search strategy may have missed some relevant studies. Second, we excluded neonatal studies from our review given the marked differences in use of innovative ventilation technologies in this patient group. Third, whilst we used a range of strategies to identify innovative ventilation technologies (collaborator expertise, guideline review, expert advisory group) and developed a standardised definition, it is possible that other experts might have recommended the inclusion of different technologies, such as tracheal tubes with sub-glottic suction.
In conclusion, our scoping review which charted evidence 851 studies across 22 innovative technologies identified marked variability across technologies in the type and volume of evidence available to inform clinical decision-making. For most technologies, there remains a need for further high-quality multi-centre clinical trials that report patient-focussed outcomes.
ICU: Intensive Care Unit
PRISMA-ScR: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews
This scoping review used only published evidence that was already in the public domain. There was no requirement for any ethical or regulatory review.
Open Science Framework: Supplementary information for "Innovative ventilation technologies used in the intensive care unit for adults and children: a scoping review’’ https://osf.io/szkqn/37
This project contains the following extended data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Open Science Framework: PRISMA-ScR checklist for ‘Innovative ventilation technologies used in the intensive care unit for adults and children: a scoping review’ https://osf.io/szkqn/37
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
AE, RC, AG, PA, DG, JY, DFM, GDP, BRS, MT, YFC, KC conceptualised the study. RC developed and ran the database searches. AE, AJB, MB, DC, GC, CMH and KC screened titles and abstracts. Full texts were reviewed by AE, MB, DC, GC and CMH. Data extraction was carried out by AE, AJB, DC, AB, MZ, and KC. AE, RC, YFC and KC drafted the manuscript. All authors critically revised the manuscript and approved the final version. AE, YFC and KC had full access to study data. KC and YFC supervised the study.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical Intensive Care
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Stock MC, Downs JB, Frolicher DA: Airway pressure release ventilation.Crit Care Med. 1987; 15 (5): 462-6 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Critical Care Medicine, Mechanical Ventilation, Artificial Intelligence.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Jan 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)