Keywords
Target product profiles, diagnostics, infection, exacerbation, unmet needs, cystic fibrosis
Infections and exacerbations are common for people living with Cystic Fibrosis (pwCF). This project aimed to develop patient-focused diagnostic target product profiles (TPP) to address unmet diagnostic needs for pwCF with international relevance.
This project involved three phases, guided by an expert advisory group of key opinion leaders (KoLs) including methodologists, pwCF, and clinical staff, and including lived experience perspectives at every stage.
Phase 1 involved a landscape analysis to identify and prioritise unmet diagnostic needs via focus groups with clinical experts and pwCF, as well as a scoping review of the diagnostic space and available diagnostic tests.
Phase 2 involved drafting the TPPs using evidence from existing literature and regulatory documentation, focus groups, one-to-one interviews with KoLs, and web-based surveys, to define ‘minimal’ and ‘optimal’ characteristics for each TPP.
Phase 3 refined and validated the TPP content through additional interviews, a two-round modified Delphi exercise, and a virtual symposium.
A comprehensive document detailing four TPPs was drafted and released freely for use. The document includes aspirational TPPs covering diagnostics for managing acute pulmonary exacerbations, rapid pathogen identification, and antimicrobial susceptibility tests, as well as a detailed TPP defining in-vitro diagnostic tests for rapid detection of non-tuberculous mycobacteria (NTM) pulmonary infections. The document contains additional guidance for diagnostic development and research needs identified for pwCF.
The project consulted with over 150 individuals and experts in CF infection management, and diagnostic development. The TPP guidance document supports research and development of patient-focused diagnostics for the benefit of pwCF.
People with cystic fibrosis (CF) need to keep their lungs as healthy as possible to stay well. To do this, it is important to spot and treat lung infections and "flare-ups", when symptoms suddenly worsen, at an early stage. Doctors use medical tests to find out if someone is developing an infection or flare-up, but these tests aren't perfect - some take too long to be useful, some of them fail too often, and some can only be used with mucus samples that not all patients can produce.
To help improve the health of people living with CF, we need better, faster, and easier tests to find lung infections and support doctors in their decisions and treatments.
This project involved doctors, scientists, and people with CF coming together to design a set of goals and guidelines for new medical tests, called Target Product Profiles (TPPs). By following these guidelines, companies and researchers making new tests will know exactly what patients and doctors need the tests to do in order to best support people with CF.
After speaking with more than 150 different people with experience of CF, the project team discovered that the main problems that people with CF wanted us to solve were:
- Slow test results from current lab methods.
- A need to spot signs of flare-ups as early as possible.
- Difficulty in choosing the right treatment for an infection.
- Dealing with hard to detect bugs like Nontuberculous mycobacteria (NTM), which are difficult to identify with current tests.
The team then worked with these experts to develop a TPP booklet that describes clearly what an ideal test should do, and what doctors and patients consider to be the lowest and highest acceptable requirements for these tests.
This paper explains how the project team completed this study, so that other researchers can learn the process and use it in other settings.
Target product profiles, diagnostics, infection, exacerbation, unmet needs, cystic fibrosis
 How to cite: Green K, Howe N, Takawira C and Holmes R. The development of patient-focused diagnostic target product profiles for detection of infection and exacerbation in people with cystic fibrosis [version 1; peer review: 1 approved]. NIHR Open Res 2025, 5:112 (https://doi.org/10.3310/nihropenres.14054.1)
First published: 24 Nov 2025, 5:112 (https://doi.org/10.3310/nihropenres.14054.1)
Latest published: 24 Nov 2025, 5:112 (https://doi.org/10.3310/nihropenres.14054.1)
How to cite: Green K, Howe N, Takawira C and Holmes R. The development of patient-focused diagnostic target product profiles for detection of infection and exacerbation in people with cystic fibrosis [version 1; peer review: 1 approved]. NIHR Open Res 2025, 5:112 (https://doi.org/10.3310/nihropenres.14054.1)
First published: 24 Nov 2025, 5:112 (https://doi.org/10.3310/nihropenres.14054.1)
Latest published: 24 Nov 2025, 5:112 (https://doi.org/10.3310/nihropenres.14054.1)
- Developed patient-focused diagnostic target product profiles (TPPs) to address unmet diagnostic needs for people with Cystic Fibrosis (pwCF), accessible here: Patient-Focused Target Product Profiles for CF-Related Infections
- Conducted novel methodology in three phases involving landscape analysis, drafting TPPs, and refining/validating TPP content through expert consultations and a consensus exercise utilising modified Delphi methodology.
- Created four comprehensive TPPs for managing acute pulmonary exacerbations, rapid pathogen identification, antimicrobial susceptibility tests, and rapid detection of non-tuberculous mycobacteria (NTM) pulmonary infections.
- Engaged over 150 individuals, including clinical experts, methodologists, and pwCF, to ensure the TPPs were patient-focused and based on clinical need.
- The TPP guidance document supports the development of patient-focused diagnostics, benefiting pwCF and CF healthcare providers.
- This project represents the close collaboration between research methodologists (NIHR HRC), UK national centre for medicine research and innovation (Medicines Discovery Catapult), and medical research charities (LifeArc and the CF Trust).
Optimising and preserving lung function is key to maintaining good clinical outcomes for people with cystic fibrosis (CF)1,2. Timely detection and clinical management of exacerbations and infections is central to achieving this aim, preventing disease progression, and minimising further complications.
According to the British In Vitro Diagnostics Association (BIVDA), diagnostic tests are used in approximately 70% of all clinical decisions, with diagnostic activities accounting for 85% of NHS clinical pathways3. The importance of diagnostic confirmation for complications of CF is reflected in the National Institute for Health and Care Excellence (NICE) national guidelines for management of pulmonary infection in CF4. Considering the James Lind Alliance (JLA) 2022 refresh of CF research priorities identified the second most prioritised research question as ‘What is the best way to diagnose lung infection when there is no sputum, for example children and those on modulators?’5, the significance of appropriate, accurate, timely, and acceptable diagnostics is of paramount importance to the clinical management of pwCF. In addition to impacting outcomes, precise diagnostics support tailored treatment strategies and inform clinical management, thereby supporting the preservation of lung function in pwCF.
CFTR modulator therapy has introduced a new ‘normal’ for people with CF, positively reducing the frequency and severity of exacerbations6, but also altering the clinical presentation of infections in many people with CF, making timely identification of deterioration in health more difficult for patients and clinical care teams alike. This changing landscape has also revealed new diagnostic unmet needs and presents a need for the CF community and diagnostic developers to work together to support the development of patient-centred diagnostics for lung infections. Despite improvements to the overall health of many pwCF, there remains a number of diagnostic challenges regarding symptom variability, the clinical significance of some pathogens, poor sample availability, and culturing delays.
This work, conceived by the CF Antimicrobial Resistance (AMR) Syndicate (cfamr.org.uk), and delivered in partnership with the Newcastle NIHR HealthTech Research Centre: Diagnostic and technology evaluation (NIHR HRC DTE), builds on the patient-centric methodology and network of the CF AMR Syndicate in the development of TPPs to guide CF antimicrobial therapeutic development7. This work outlines the process for conception, refinement, and validation of a series of patient-focused diagnostic Target Product Profiles (TPPs) aiming to address prioritised unmet diagnostic needs for people with CF.
This body of work involved three study phases overseen by an independent expert advisory group, as displayed in Figure 1. The project duration was 24 months from initiation to finalised Target Product Profile (TPP) document, available here: Patient-Focused Target Product Profiles for CF-Related Infections
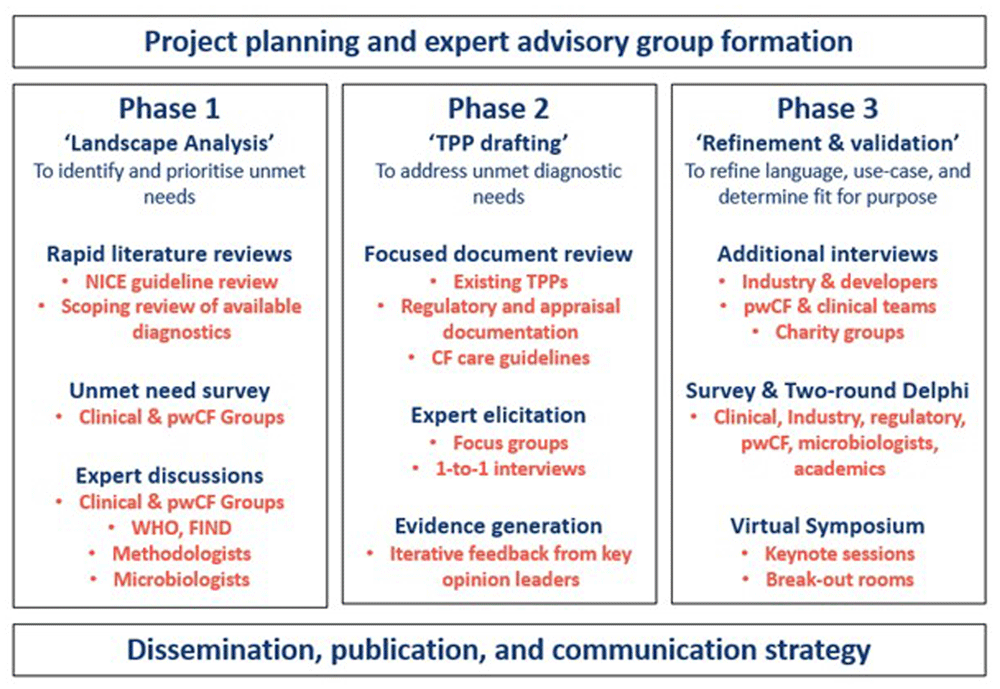
This study was conducted in three main phases – Phase 1 covered the initial landscaping work to identify and prioritise the unmet diagnostic needs in CF. Phase 2 build on these prioritised needs by drafting initial TPP characteristic tables through literature review, expert elicitation, and other evidence generation techniques. Phase 3 involved refinement and validation of the TPP drafts to develop a finalised TPP document. The project had oversight from an external advisory group who provided feedback and direction throughout.
PPIE activities were incorporated throughout this project, from project planning through to delivery and included both public contributors and people with Cystic Fibrosis (pwCF). Initial project design was reviewed by the NIHR HRC DTE ‘Insight Panel’ of public contributors prior to initiation of the work. The ‘Insight Panel’ were approached at strategic milestones in the project to review and advise on next steps including at each phase of the project (see Figure 1 below).
An independent external advisory group (EAG) was brought together for this project which included pwCF and public representatives as well as clinical, academic, laboratory, and methodology experts. The EAG were consulted at the drafting stage, at each phase, and during the elicitation and dissemination stages to provide advise and feedback on progress and direction.
Informed consent forms, project outlines, and participant information sheets were drafted jointly by the CF AMR Syndicate and NIHR HRC DTE with some revisions following consultation with a patient and public involvement and engagement (PPIE) manager and PPIE engagement group. The virtual symposium held after the focus groups had a pwCF as a keynote speaker and pwCF in all breakout room and sessions.
Our expert advisory panel consisted of eight experts: a person with lived experience of CF, a paediatric and an adult CF physician, a methodology expert in TPP development, a representative from a diagnostics trade association body, and two academics with an interest in CF and microbiology. This panel met formally seven times over the project lifecycle, supplemented by ad-hoc meetings and online document review.
Methodologists experienced in guideline review searched and summarised NICE guidelines [NG78, QS168, NG237] relating to CF infection and exacerbations to build an understanding of the care pathway and current practice in the UK NHS.
A scoping review was conducted to identify tests (both currently in use or available on the market, and those still in development, published in the last 10 years) that diagnose and predict respiratory infections and exacerbations. In doing so, we could assess the current landscape of diagnostic tests available for use, identify diagnostic gaps, and identify major developers working in this space. This analysis would inform the future development of novel diagnostic tests in this area with further information on the methodology and output of this report noted in the publication8.
Diagnostic test characteristics and input values were extracted and collated from relevant existing and published TPP documents hosted by established TPP developers [Appendix 1]. TPPs act as blueprints and targets for developers to build against, ensuring that products are designed to provide clinical utility or address an unmet need. These were used to underpin and categorise sections of our draft diagnostic TPP document and used as the basis of prioritisation discussions during early expert elicitation stages.
Key CF literature including standard of care guidelines and laboratory guidance documents for processing microbiological samples were used to build and guide the clinical context as well as the key considerations conveyed throughout the document.
Informed consent forms, project outlines, and participant information sheets were drafted by all participating organisations, in collaboration with the PPIE team.
Separate draft focus group schedules were designed for clinical staff and CF patients by members of the Newcastle NIHR HRC DTE and revised iteratively through consultation with the wider project team. Three focus groups were conducted via Microsoft Teams totalling 12 clinical participants and six people with lived experience, lasting between 90 and 120 minutes. Recorded sessions were transcribed via otter.ai before being cleaned and checked for accuracy by an experienced methodologist. Notes were taken by supporting researchers throughout the session and used to assist the transcription and analysis. Accuracy of the interview content was confirmed by the methodologists, and any outstanding issues or clarifications were resolved with the focus group interviewees.
Additional interviews were conducted with individual stakeholders from within and outside of the UK. These included methodologists in TPP development, members of the world health organisation, innovators from industry, academics with expertise in CF microbiology, microbiologists, paediatric and adult CF physicians, laboratory staff, were conducted following the methods outlined above with a stronger focus on eliciting specific information relevant to the TPP development process.
Following data collection, rapid thematic analysis was performed based on the recordings, transcripts and notes taken during the interviews and focus groups. A thematic summary document was produced, and its contents were checked for accuracy and completeness by the methodologists and wider project team involved.
The project team disseminated a survey to rank prioritise 10 options for potential TPP development based on the unmet needs identified in phase 1 focus group interviews. The survey, written in SurveyMonkey, was open for 2 weeks, from Friday 12th May 2023 – Friday 26th May 2023. It was sent to selected individuals including members of the TPP expert advisory group project, clinicians, industry liaison, researchers, people with CF and their loved ones. Participants provided their ranking according to ‘priority’ and ‘feasibility’.
For the rank prioritisation exercises, a 10-point Likert scale was used to weight the responses, ranging from 1 (lowest priority) to 10 (highest priority). The rating average was a mean, calculated as follows, where w = weight of answer choice, x = response count for answer choice:
(x1w1 +x2w2 + x3w….xnwn)/total responses
The project team disseminated a survey to gather opinions and formulate consensus on TPP topic selection, validation of the prioritised unmet needs, key considerations, and value propositions collated from phase 1 and 2, and to determine the level of agreement in the CF community with regard to the proposed TPP content. The survey, written in SurveyMonkey, and composed of 12 questions, was open for 3 weeks from 3rd October 2023 to 23rd October 2023, resulting in 102 total responses (note that some responses were partially complete - total number of responses therefore varied by question). The survey link was distributed across the globe through established networks of the NIHR HRC DTE, clinical care teams (covering physiotherapy, nursing, physician, and pharmacy networks), pwCF, industry, microbiology and academia. It utilised a 5-point scale from ‘strongly disagree’ to ‘strongly agree’ with an N/A option and compulsory free-text box to explain the reasoning for the selection. A thematic analysis was performed on the qualitative responses to the survey and themes agreed by consensus amongst the project team’s qualitative expert methodologists. [Appendix 1]
We used a 2-round modified Delphi approach with an 80% consensus threshold to determine consensus on non-tuberculous mycobacterium pulmonary disease (NTM-PD) amongst NTM experts, on the NTM TPP. Thirty-five NTM-PD experts including healthcare professionals and academics participated, recruited from the NTM Network UK [https://www.ntmnetworkuk.com/]. Round 1 comprised of 28 questions and was open to input for 3 weeks, between 25th October 2023 and 12th November 2023. Round 2 comprised of 22 questions, and was open for input for 2 weeks between 13th November 2023 and 1st December 2023.
A thematic analysis was performed on the qualitative responses to the Delphi, and themes agreed by consensus amongst the project team’s qualitative expert methodologists. Wording suggestions and changes were incorporated between Round 1 and Round 2 of the Delphi exercise. [Appendix 2] [Appendix 3]
The CF AMR Syndicate hosted a virtual symposium on 6th of December 2023. Convening diverse, international expertise from the clinic, academia, industry, and people with CF, the event provided a platform to discuss key challenges in current infection and exacerbation diagnostics in CF and identify opportunities to address them. More information on the virtual symposium and outputs can be found here: https://cfamr.org.uk/report/virtual-symposium-on-diagnostics-meeting-report/
Literature reviews from Medline and Embase databases identified 2133 references, of which 1184 were screened after de-duplication. 261 papers met the inclusion criteria based on title and abstracts, and 200 were included after full text screening8. Approximately 100 different tests and modalities were identified and grouped according to use-case and sample type. This review provided the base knowledge of available diagnostics tests for infections and exacerbations in CF, supporting phase 2 TPP drafting.
Key themes from phase 1 focus groups included the need for more acceptable sampling methods (including non-sputum sampling), greater patient acceptability - particularly for sampling and convenience of testing, a need for faster time to results to inform treatment and reduce the reliance on empirical antibiotic use, and a need for novel tests to have demonstrable clinical relevancy, with a preference for accuracy over speed in non-acute settings.
Approximately 10 unmet diagnostics needs were identified from initial clinical and pwCF focus groups, under three primary categories: Baseline monitoring and detection of exacerbations, pathogen identification, and treatment monitoring. These unmet needs were prioritised by 15 clinical, laboratory, academic, and industry contributors, and 9 people with lived experience of CF based on the urgency of the unmet need, and the feasibility in finding a solution. The rankings (Table 1) were used to support prioritisation of TPPs for phase 2.
This table outlines the prioritised unmet diagnostic needs derived from clinical and ‘lived experience’ focus groups ranked by greatest need, and most feasible to address, with ‘1’ being the ‘greatest need’ and ‘most feasible’ respectively.
The study team, guided by the expert advisory group, and the results of the prioritisation exercise, selected the TPP topics outlined in Figure 2. The chosen TPP topic areas included generic testing needs for people with CF, and specific TPPs for 1) monitoring and predicting pulmonary exacerbation, 2) rapid pathogen detection and identification, 3) antimicrobial susceptibility testing, and 4) rapid diagnosis of NTM pulmonary infections.
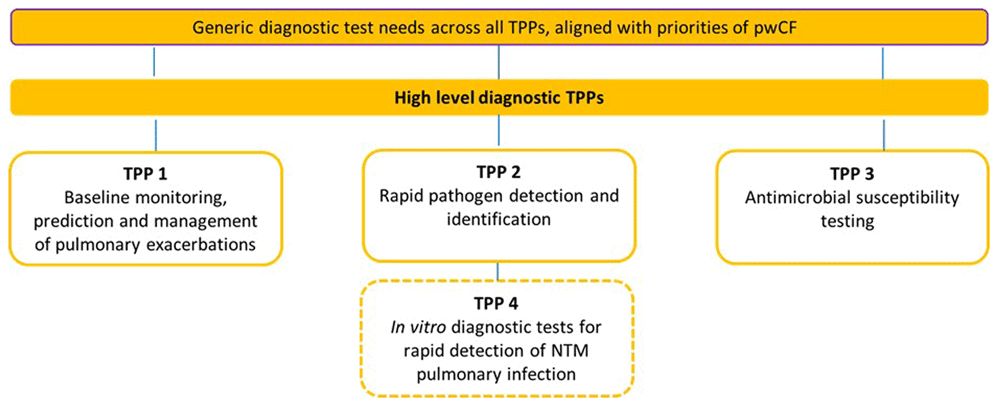
Figure 2 shows an overview of the suite of TPPs developed in this project. 4 TPPs were generated along with additional generic content describing unmet diagnostic needs and priorities of people with Cystic Fibrosis. TPP1-3 were ‘high-level’ TPPs aiming to provide an overview of characteristics to address – TPP1 – baseline monitoring, prediction, and management of pulmonary exacerbations; TPP2 – Rapid pathogen detection and identification; and TPP3 – Antimicrobial susceptibility testing. A more in-depth ‘full TPP’ was developed for TPP4 – in vitro diagnostic tests for rapid detection of NTM pulmonary infection.
A review of typical test characteristics in established TPPs was performed. 57 TPPs were screened [Appendix 4], and their characteristics were extracted to inform the study team and expert advisory group’s decision making.
Each TPP produced in this study included:
An introduction with a primer on the topic - clinical presentation, management, prevalence, current testing modalities, and the unmet need, as informed by relevant literature and standards of care guidelines
Value Propositions describing the added value a test would need to demonstrate.
Key considerations underpinned by expert-elicited information and literature relevant to the unmet need.
Table of characteristics with minimally acceptable, and optimal test characteristics as determined by key opinion leaders in CF-AMR and diagnostic development organised into 5 categories covering scope, design, performance, operational considerations, and other product considerations.
TPP 1-3 do not contain all characteristics as the aspirational nature of these TPPs precluded detailed descriptive requirements.
The TPP content, including the minimal and optimal requirements and potential value propositions, were derived from focus groups, 1-to-1 clinical, industry, and lived-experience interviews, utilising language and content from previously published TPPs identified in Phase 1.
‘Minimal’ requirements were defined as the lowest possible threshold to provide clinical utility, based on the value propositions highlighted during by expert interviews with key opinion leaders. ‘Optimal’ requirements were defined as values offering the best clinical utility based on the value propositions highlighted in the TPP as determined by expert interviews with key opinion leaders (Table 2).
This table displays the table of characteristics for one of the developed TPPs, explaining the content the table covered and the purpose and need for that characteristic to be defined and included in the TPP. As each TPP table differed slightly based on context and need, this table is based on TPP4, the most comprehensive TPP developed in this project.
The TPP content was refined through discussion with key stakeholders, a two-round modified Delphi consensus survey, and a virtual symposium that featured ‘lived experience’ perspectives, industry, academia and clinical discussions, and breakout rooms answering key questions.
All of the TPP content for the high level TPPs (TPP1-TPP3), including the elicited value propositions, key considerations, and characteristic values were incorporated into a refinement survey to assess community agreement and elicit feedback. The survey was completed by 102 individuals, including 21% CF physicians, 10% physiotherapist in CF, 12% CF nurse specialists, 15% CF academics, and 11% people with lived experience of CF. Over 85% of all responses were ‘Agree’ or ‘Strongly Agree’ across each of the TPPs regarding the value propositions and TPP characteristics (Figure 3a and Figure 3b). Respondents were invited to provide commentary on the rationale of their response which was used to refine the TPP document further.
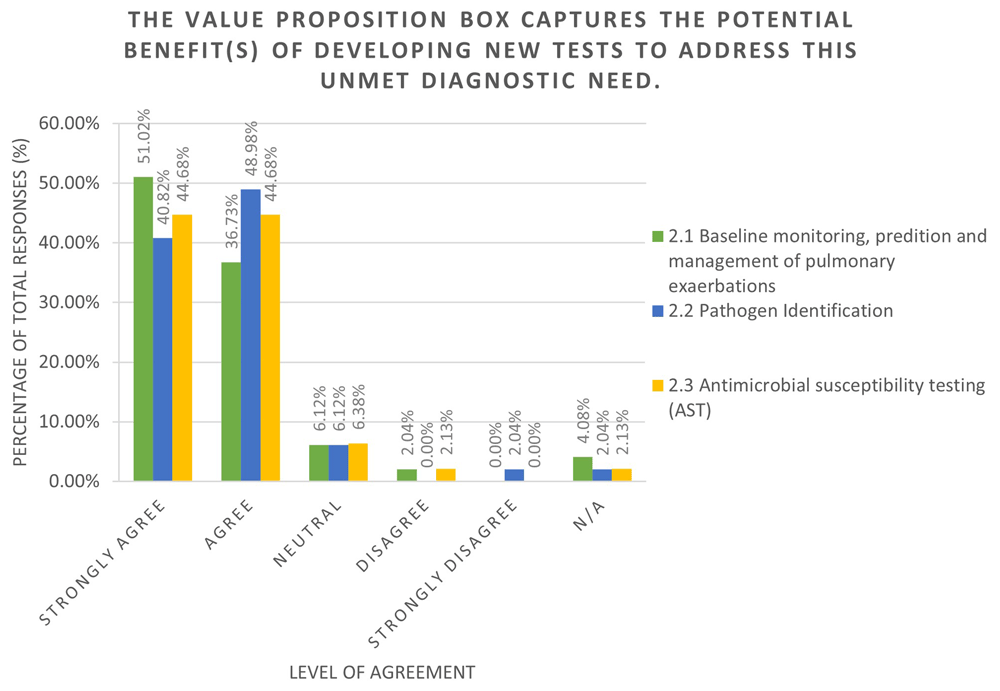
Figure 3.a displays the overall responses and level of agreement for each of the TPPs covered in the high-level TPP survey. This question covered the value propositions outlined in the TPP document. The Value propositions for all three TPPs included in the survey were positively received, indicating strong agreement with the TPP content. The N/A option was used in the survey for both 'I don't feel qualified to answer' and 'I do not wish to answer' responses.
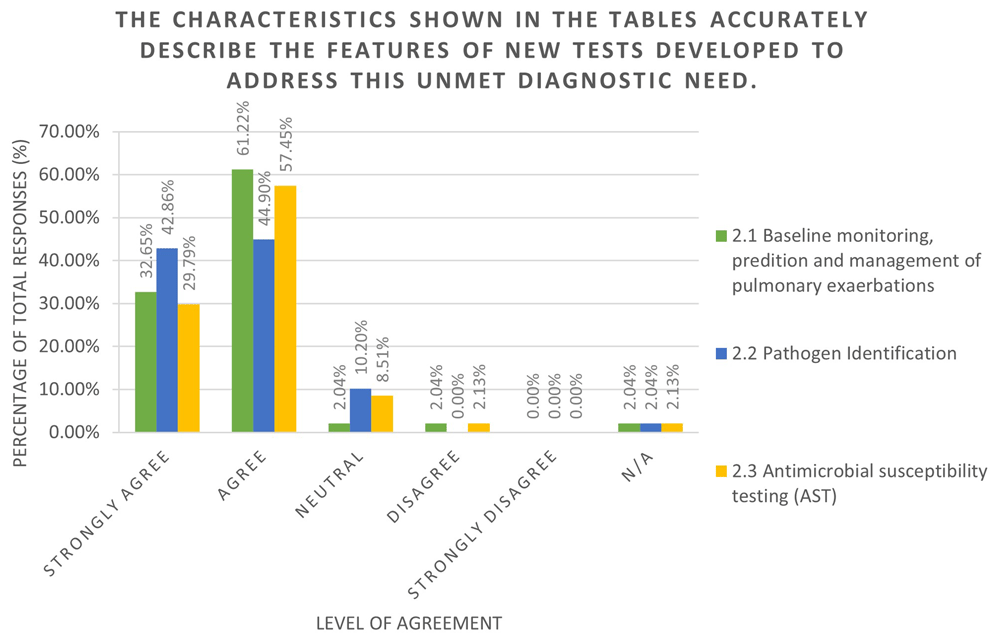
Figure 3.b displays the overall responses and level of agreement for each of the TPPs covered in the high-level TPP survey. This question covered table of characteristics outlined in the TPP document. The tables of characteristics for all three TPPs included in the survey were positively received, indicating strong agreement with the TPP content. The N/A option was used in the survey for both 'I don't feel qualified to answer' and 'I do not wish to answer' responses.
Based on the initial focus groups and expert interviews in phase 1 and 2, additional sections were added to inform users of the TPP about CF-specific issues. These included the impact of modulator therapies, sample considerations due to reduced sputum production, the needs and priorities of people with CF, and considerations for diagnostic developers, including the need for robust analytical validity studies and health economic analysis to drive adoption and implementation.
For TPP4, a consensus study was designed using a modified 2-Round Delphi approach. Round-1 of the Delphi survey achieved above 80% consensus, with an average of 95% positive agreement. Open-text responses indicated a need for wording clarifications and highlighted additional functionality of the tests.
Round-2, completed by 26 participants, sought consensus agreement on the revised wording from round-1. Round-2 also achieved above 80% consensus, with an average of 92%. No additional rounds were required.
The final method of expert input and consultation was a virtual symposium. Outputs from this symposium were incorporated into the revised TPP document before final confirmation by the project team and advisory committee, and subsequent publication.
The final TPP document included one general table defining the diagnostic testing preferences and considerations of pwCF, three TPPs to address aspirational requirements (TPP1-3), and one detailed TPP defining a rapid test for NTM-pulmonary disease (NTM-PD) (TPP4).
This project sought to develop a suite of patient-focused target product profiles to address previously undefined diagnostic unmet needs for lung CF infections. Elicitation of unmet needs from pwCF and clinical focus groups centred on the time to results for pathogen detection by current standard microbial culture methods, the definition and early detection of exacerbations, the lack of clinical utility for current culture-based anti-microbial susceptibility testing methods, and the need for rapid and reliable detection of slow-growing and difficult to culture organisms such as NTM. These topic areas formed the basis of the TPP document. Together, this suite of patient-centred TPPs define the optimal and minimal requirements and test characteristics as defined by a broad range of clinical and lived-experience CF experts to address the outlined unmet diagnostic needs.
The needs and priorities elicited in this project, and contained in the TPP document and associated qualitative publications, should be considered a ‘starting-point’ for diagnostic developers to inform research and development efforts, but should not be used in place of prospective qualitative elicitation activities with individuals representing an appropriate, diverse selection of the CF community. When using these TPPs to inform design and development, test developers may need to balance and prioritise design characteristics based on the expected value propositions and clinical impact expected of the device. It is important to consider and align with the priorities of pwCF, as likely end users of a device - diagnostic accuracy, time to result, sample type, target setting, and time to results were considered priority characteristics through elicitation in this work.
For example, in terms of acceptability of testing and sampling for pwCF, there was a clear preference towards at-home and community testing to reduce the burden of outpatient appointments and minimise disruption to work, school, and life. Some testing modalities may lend themselves more easily to at-home testing, or self-testing, but laboratory-based tests may align with these preferences if sample collection can be performed at-home or in a community setting and sent to a clinical laboratory. Both pwCF and clinical care teams noted that advantages in ease of use should not compromise accuracy or reproducibility of a test.
With the publication and dissemination of this suite of target product profiles incorporating the opinions and preferences of over 150 key opinion leaders in CF-AMR the CF AMR Syndicate, NIHR HRC DTE, and collaborators have distilled the optimal and minimal characteristics for devices aiming to address diagnostic unmet needs as outlined by the wider CF community. By utilizing the information contained in the TPP document, and the associated publications, developers should be well informed to steer product research and development in this space towards improvement of clinical care and management outcomes for people with CF.
The suite of Target Product Profiles has been published on the CF AMR Syndicate website9. Other aspects of this project have been published separately, including the diagnostic landscape scoping review, unmet needs elicitation exercise, and summary report for the virtual symposium8,10. By making these outputs publicly available the project team aims to influence: developers in the development of their diagnostics in the CF and adjoining respiratory infection space, policy makers and regulatory bodies in the guidance and policies for CF infection management; TPP methodologists to further develop the TPP development process and further incorporate lived experience into the TPP profile and; charities, funders, and government bodies to capitulate on the work outlined here to drive greater, targeted funding to address the unmet diagnostic needs in CF.
The initial focus group activities described in phase 1 were authorised as part of a service evaluation approved by Newcastle upon Tyne Hospitals Trust in May 2022, prior to the focus group taking place (project number: 13687).
The subsequent activities for this project were registered through Integrated Research Application System (IRAS) - Reference number: 316276. Ethical approval was granted through Favourable Opinion of the Cambridge Central Research Ethics Committee and the project received a letter of approval on 18th October 2022 to cover interviews, questionnaires, Delphi’s and focus groups with NHS clinical staff.
All identifiable information collected during the elicitation process was anonymised prior to reporting. For recording of the focus groups, consent was confirmed verbally by the participants prior to starting the focus group and witnessed and recorded on the consent form by a second researcher. Verbal consent was thought to be most appropriate as the focus groups were conducted remotely and this approached placed least burden on participants. The collection of verbal consent was approved as part of the IRAS application and service evaluation.
Underlying data is available via Newcastle University data repository:
Data directly linked to this manuscript, including the phase 2 and phase 3 refinement surveys and Delphi methodology and the full Table 2 data.
Data available at: https://doi.org/10.25405/data.ncl.29911967.v2
Data file 1 – Question set and raw results for the High Level (TPP1-3) survey.
Data file 2 – Question set and raw results for round 1 of the Delphi survey.
Data file 3 – Question set and raw results for round 2 of the Delphi survey.
Data file 4 – Full TPP (TPP4) table of characteristics with reasoning and purpose of including each characteristic. Referred to as ‘ Table 2 – Table of Characteristics based on the NTM TPP (TPP4)’ in this manuscript.
Data are available under the terms of the Creative Commons Attribution 4.0 International (CC-BY-4.0).
Data linked to Phase 1 of this study.
Data available at: https://doi.org/10.25405/data.ncl.26477263.v1
Data file 1- Clinical focus group topic guide
Data file 2- pwCF focus group topic guide
Data file 3- Diagnostic TPPs lay summary
Data file 4- Information for clinical team focus group
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Transcripts from Phase 1 scoping focus groups.
Data available at: https://doi.org/10.25405/data.ncl.26565871.v1
Data file 1- Clinical Focus Group 1 transcript
Data file 2- Clinical Focus Group 2 transcript
Data file 3- Patient focus group transcript
Data are available under the terms of the Creative Commons Attribution 4.0 International (CC-BY-4.0).
The authors would like to thank LifeArc, Cystic Fibrosis Trust and the Medicines Discovery Catapult (managing partners of the CF AMR Syndicate) for their discussions and support for this work. The Authors would also like to thank individuals who contributed to the elicitation, survey and symposium activities, the expert advisory group for this project and PPIE members from the Newcastle NIHR HRC DTE who contributed to the development of the participant facing documents and provided feedback on the project plan and outputs.
The authors also acknowledge the ongoing support and contributions of the Newcastle HRC DTE and the NIHR. The views expressed in this manuscript are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.

Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: My research mainly focuses on CF clinical care.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 24 Nov 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)