Keywords
Breast cancer, physical activity, exercise, referral pathway, intervention development, quality of life,
Breast cancer and its treatment can have long-term adverse effects on physical and mental health. Evidence-based guidelines recommend that healthcare professionals (HCPs) advise women with breast cancer to engage in physical activity to improve health outcomes. However, support to be active is not standard care. The PURE-EX (EXpanding into communities to imProve physical activity sUpport foR womEn after breast cancer) programme aims to address this knowledge-practice gap.
To co-develop a programme that integrates physical activity referrals into standard care for women after treatment for early-stage and locally-advanced breast cancer. Programme components will include:
A referral pathway enabling HCPs to refer women to community-based physical activity programmes after they have completed primary treatment for breast cancer;
An online training course to support community providers in adapting their existing physical activity programmes for women who have undergone breast cancer treatment.
We will co-develop, refine, and evaluate PURE-EX programme components through four work packages (WPs):
WP1. Conduct a systematic scoping review to describe the characteristics of community-based physical activity programmes for women with breast cancer reported in the literature.
WP2. Undertake qualitative research with: (i) women with breast cancer, (ii) HCPs responsible for their care, and (ii) exercise professionals, to explore barriers and facilitators to incorporating physical activity into breast cancer care from different perspectives.
WP3. Hold co-development events to develop and refine components of the PURE-EX programme and gain insights as to how it could be operationalised in practice.
WP4. Conduct a feasibility trial in 45 women who have finished primary treatment for breast cancer to assess the feasibility and acceptability of the PURE-EX programme.
The PURE-EX programme will be an evidence-based, theory-informed, and person-centred intervention, with the potential to make physical activity support routinely available for women after breast cancer treatment.
More women are surviving breast cancer than ever before. But women often experience low mood, reduced strength and fitness, and extreme tiredness. These issues can last for years after treatment.
Being active after breast cancer treatment can help. It can make women feel less tired, help them return to full strength, and improve their overall health and wellbeing. It may also lower the chances of cancer coming back.
Unfortunately, most women do not get support to be active after treatment. The main issue is that there aren’t enough suitable physical activity classes for women to join. There also are no easy ways for healthcare workers to refer women to classes.
We want to develop a programme that allows women to be referred to suitable physical activity classes after their treatment. To do this, we will:
Create a new pathway for healthcare workers to refer women to local physical activity classes.
Develop an online training course to help community providers adapt their classes for women who have had breast cancer treatment.
Stage 1. We will start by looking at existing studies to see what community physical activity classes have been delivered for women with breast cancer.
Stage 2. Next, we will talk to three groups of people: (a) women with breast cancer, (b) healthcare workers responsible for their care, and (c) exercise instructors. We want to understand what helps women stay active after treatment.
Stage 3. We will hold workshops with women, healthcare workers, and exercise instructors. Together, we will create the referral pathway and online training course.
Stage 4. Finally, we will test the programme with 45 women who have just finished their breast cancer treatment. We want to find out if they liked the programme, if it helped them, and how we can improve it.
Breast cancer, physical activity, exercise, referral pathway, intervention development, quality of life,
Breast cancer is the most common cancer in the UK. Around 58,800 women are diagnosed with breast cancer each year, accounting for 13% of all new cancer cases1. Approximately 90% of breast cancer cases are diagnosed at an early or locally advanced stage (stages I–III).
Survival from breast cancer has doubled in the last 40 years in the UK due to advances in screening, early detection, and treatment2. In 2020, the 5-year survival rates for early-stage and locally advanced breast cancer were 98% for stage I, 90% for stage II, and 72% for stage III3. This has led to a significant increase in the number of breast cancer survivors, with an estimated 600,000 women currently living in the UK after a breast cancer diagnosis. This number is predicted to rise by approximately 10% each year, reaching 1.7 million by 20404.
Breast cancer and its treatment can have long-term adverse effects on both physical and mental wellbeing. Women diagnosed with breast cancer report lower quality of life (QoL) during treatment, and for at least 1-year afterwards, compared with women with no history of breast cancer5,6. Almost half (48%) of women diagnosed with early-stage breast cancer experience depression or anxiety in the year after diagnosis7. Cancer-related fatigue persists in around one-third of women for 5 to 10 years post-treatment8, which can contribute to difficulties in returning to work and living independently9. Cardiorespiratory fitness and muscle strength, which are strong predictors of all-cause and cancer-specific morbidity and mortality10–12, are 20–25% lower in women after breast cancer treatment compared with sedentary women with no history of cancer13. Breast cancer survivors also have increased risk of developing non-breast primary cancers14 and other chronic health conditions, including type II diabetes15 and cardiovascular disease16. Therefore, strategies are needed to address the physical and psychosocial sequelae of breast cancer and its treatment.
Guidelines issued by several expert bodies, including the American Society of Clinical Oncology (ASCO)17, National Comprehensive Cancer Network (NCCN)18, and American College of Sports Medicine (ACSM)19, recommend that healthcare professionals (HCPs) advise people diagnosed with cancer to engage in regular physical activity to mitigate the side effects of treatment. This recommendation is based on strong evidence from randomised controlled trials that physical activity interventions improve QoL, fatigue, cardiorespiratory fitness, muscular strength, and symptoms of anxiety and depression in people with cancer, including breast cancer17,19. Additionally, recent findings from the Global Cancer Update Programme showed that participating in the highest versus lowest amounts of leisure-time physical activity after a breast cancer diagnosis was associated with a 44% lower risk of all-cause mortality and 42% lower risk of breast cancer-specific mortality20.
Support from HCPs is crucial because physical activity levels decline during breast cancer treatment and do not return to pre-treatment levels21. Self-reported UK data show only 20% of breast cancer survivors are regularly physically active22,23 and 28% are completely inactive22. Most women want to receive physical activity guidance from their breast cancer care team23–25, and an oncologist’s recommendation to exercise increases exercise levels in women during and after treatment26,27.
Despite evidence-based guidelines, support to be active is not standard care. Our UK-wide survey23 showed one in five (20%) women with breast cancer recall receiving physical activity advice after completing treatment, and only 10% were referred to another source of information or physical activity specialist. These findings align with other UK-based data showing only 9% of cancer nurses report talking to patients about physical activity28. HCPs report many barriers to providing physical activity support, including organisational-level barriers such as limited resources (e.g., staff, funding), absence of a referral pathway, and a lack of suitable physical activity programmes to refer to29–32. HCPs also report individual barriers, such as having a lack of time, inadequate knowledge of physical activity, and not being the right person to give advice29,30,33.
Efforts to make physical activity support standard practice in oncology have been proposed, most notably through the USA-based ‘Moving Through Cancer’34,35 initiative. This initiative draws on the 3A’s approach (Ask, Advise, Act) - originally developed for smoking cessation36 - to provide HCPs with a framework to ask patients about physical activity, advise them to be active, and offer referral to community-based programmes. This approach is appealing because it can be delivered by HCPs in less than 30-seconds37 and leverages existing community resources, reducing burden on healthcare systems.
Exercise referral schemes are available in some UK regions through the National Health Service (NHS), allowing General Practitioners and allied health professionals (e.g., physiotherapists) to refer sedentary patients to physical activity programmes38. However, these schemes are not consistently available nationwide, and no referral pathway exists for breast cancer HCPs to offer women physical activity referrals32. Furthermore, most community-based physical activity programmes are not cancer-specific and are led by instructors who lack specialised training or knowledge about breast cancer. The National Institute for Health and Care Excellence (NICE) recommends that policymakers fund exercise referral schemes only if the programmes are tailored to meet individual needs38. For women with breast cancer, this requires adaptations to address or circumvent the physical and psychosocial sequelae of treatment, such as lymphoedema, arthralgia, upper-body pain and immobility, peripheral neuropathy symptoms, menopause symptoms, web axillary syndrome, and body image concerns. Having guidance from knowledgeable exercise instructors who understand issues regarding breast cancer treatment is important for encouraging physical activity participation in breast cancer survivors39.
While being active can benefit women during and after treatment19, most prefer to be active at home during treatment40 and attend an exercise facility post-treatment41–43. Evidence suggests that breast cancer survivors are more likely to seek physical activity advice after completing treatment41,44,45 with chemotherapy being negatively associated with wanting to receive advice41, likely due to treatment-related side effects46. These preferences are supported by findings from an exercise trial showing higher adherence to supervised facility-based exercise in women who had completed adjuvant treatment (83%) compared to those currently receiving chemotherapy (71%)47. Additionally, patients who initiate a physical activity programme after chemotherapy may regain cardiorespiratory fitness to the same degree as those who were physically active during chemotherapy48. Thus, the post-treatment period may be the ideal time to offer women referral to community-based physical activity programmes.
Here, we present the initial plan (pre-protocol) for the PURE-EX programme (EXpanding into communities to imProve physical activity sUpport foR womEn after breast cancer). PURE-EX will co-develop, refine, and test a programme that aims to integrate physical activity referrals into standard care for women after breast cancer treatment.
To co-develop a programme that integrates physical activity referrals into standard care for women after primary treatment for early-stage and locally-advanced breast cancer.
1. Establish a referral pathway that enables HCPs to refer women to community-based physical activity programmes after they have completed primary treatment for breast cancer.
2. Co-develop an online training course to support community providers in adapting their existing physical activity programmes for women who have undergone breast cancer treatment.
PPI has shaped this study and will remain central throughout. Feedback from women with breast cancer highlighted the lack of physical activity support. Many suggested that the end-of-treatment appointment could be an ideal time to offer physical activity referrals, as some feel a “sense of abandonment” after finishing primary treatment. Two patient representatives with lived experience of breast cancer are important members of our research team, having actively contributed to the study’s conception and design. These two representatives will co-chair a PPI panel of 10 women with varied experiences of early-stage and locally advanced breast cancer. The panel will provide ongoing PPI feedback into research team meetings as a dedicated rolling agenda item. Meeting every 3–4 months in a local café, the PPI panel will offer user perspectives across all WPs, including input on topics guides (WP2), co-development workshops (WP3), recruitment procedures and trial materials (WP4), and disseminations plans.
We intend to produce an evidence-based, theory-informed, person-centred intervention that has been co-developed with stakeholders. Aligning with guidelines for developing and adapting complex interventions49,50, we will iteratively and systematically co-develop, refine, and evaluate PURE-EX programme components – including a referral pathway and online training course for community providers – through four inter-linked work packages (WPs): systematic scoping review (WP1); qualitative research (WP2); co-development workshops (WP3); and a feasibility trial (WP4) (Figure 1). We will test and refine the logic model during the intervention development process, informed by existing models and frameworks for understanding behaviour, including the COM-B model and Theoretical Domains Framework (TDF)51,52. The logic model will outline how the intervention’s inputs, activities, and outputs are expected to improve physical activity levels in women with breast cancer, ultimately impacting longer-term outcomes, such as QoL (Figure 2).
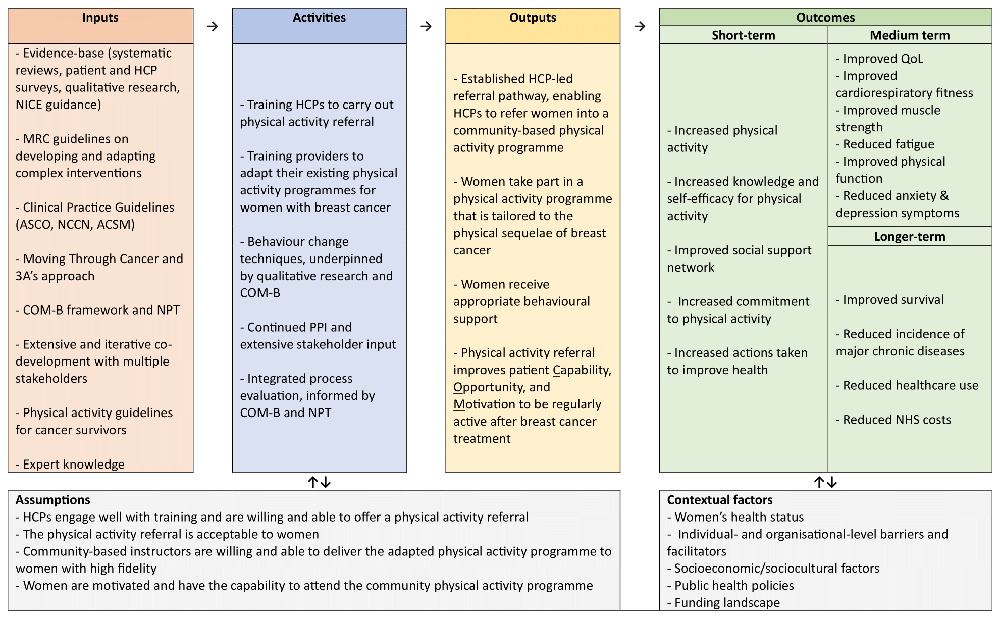
ACSM = American College of Sports Medicine; ASCO = American Society of Clinical Oncology; HCP = healthcare professional; MRC = Medical Research Council; NCCN = National Comprehensive Cancer Network; NHS = National Health Service; NICE = National Institute for Health and Care Excellence; NPT = Normalisation Process Theory; PPI = patient and public involvement; QoL = quality of life.
The aim of the referral pathway will be to enable HCPs to endorse, offer, and facilitate referrals to community-based physical activity programmes in a manner that is feasible within outpatient breast clinics. Drawing on the Moving Through Cancer initiative34,35 and 3A’s approach36, we intend for the referral to be brief (≈30-seconds) to address HCP concerns about time constraints and competing priorities32. We envisage that women who agree to the referral will have an initial consultation booked with the community provider before leaving the clinic. This approach draws on evidence from smoking cessation interventions, where uptake has been shown to be ten times higher when referrals are made at the clinic rather than leaving patients to instigate referrals themselves53. Additionally, baseline consultations with the provider may be important to the success of exercise referral schemes54,55. The process of making referrals and booking appointments will need to be compatible with, or circumvent, existing NHS IT systems. We will explore the acceptability of these methods with stakeholders and consider alternative or complementary options, such as self-referrals.
Physical activity programmes are widely available in community settings and are typically delivered by instructors with personal trainer qualifications (e.g., Level 3 Diploma in Personal Training) employed by charities, local councils, or independent leisure and fitness centres. These programmes vary but often involve group-based aerobic, resistance, or mind-body exercises (e.g., yoga), either alone or in combination. Adapting existing programmes to fit specific contexts, rather than creating new services, is both efficient and coherent with contemporary intervention development guidelines56. Thus, we intend to co-develop an online training course with the aim of helping community providers adapt their existing physical activity programmes for women with breast cancer. We envisage that the training will cover breast cancer and its treatments, the physical and psychosocial effects, behaviour change techniques (BCTs), and recommended adjustments to physical activity programmes.
Aim
To describe the characteristics of community-based physical activity interventions for women diagnosed with breast cancer reported in the literature.
Objectives
To describe:
i. The characteristics and content of community-based physical activity interventions, including approaches and processes used for intervention development and evaluation.
ii. The characteristics of women who received the interventions.
iii. Findings of intervention process evaluations in terms of recruitment, retention and adherence.
iv. The outcome domains and outcome measures that have been used to evaluate the interventions.
Design
The review will be conducted in accordance with the Joanna Briggs Institute (JBI) methodology for scoping reviews and reported in line with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR)57. The protocol was prospectively registered on Open Science Framework (OSF; https://osf.io/erjxu).
Search strategy
Two authors will independently search six electronic databases from inception: Embase (Ovid), MEDLINE (Ovid), PsycInfo, CINAHL (EBSCO), SportDiscus (EBSCO), and Cochrane Central Register of Controlled Trials (Wiley). The search string to be used in MEDLINE is displayed in Box 1, and searches for all other databases will be available on OSF (https://osf.io/xver2/). We developed the search strategy with guidance from a liaison librarian and piloted it in an initial search limited to MEDLINE and CINAHL databases. We will manually search the reference lists and forward citations of included studies and relevant reviews to identify other potentially eligible studies.
1. exp Breast Neoplasms/ or breast cancer.mp.
2. exp Exercise/
3. (exercis* or physical activ* or aerobic* or danc* or sport* or walk* or yoga or Tai Chi or pilates).mp.
4. exp Sports/
5. 2 or 3 or 4
6. community.mp.
7. exp Community Health Nursing/
8. exp Community Networks/
9. exp Community Participation/
10. (primary adj3 care).mp.
11. primary healthcare or general practice or GP or family practice or family doctor).mp.
12. exp General Practice/
13. exp Family Practice/
14. exp Primary Health Care/
15. (village hall or town hall or church or leisure centre or park).mp.
16. 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. (program* or intervention*).mp.
18. (patient* or survivor*).mp.
19. ((women adj6 breast cancer) or (individuals adj6 breast cancer) or (persons adj6 breast cancer)).mp.
20. 18 or 19
21. 1 and 5 and 16 and 17 and 20
Eligibility criteria
Original research articles will be included if they meet the following inclusion criteria: (i) participants were women diagnosed with breast cancer and aged 18 years or older; (ii) the study reports on a community-based physical activity intervention; (iii) full-text is available in English. We define community-based interventions as those delivered within a community setting such as churches, schools, town/village halls, primary care centres, leisure centres, and parks. No restrictions will be placed on breast cancer stage or treatment, study design, intervention duration, geographical location, or outcomes assessed. Exclusion criteria were: (i) participants aged <18 years; (ii) non-intervention studies (e.g., cross-sectional); (iii) interventions that did not include a physical activity component delivered in a community setting (e.g., hospitals or University laboratories); (iv) interventions that exclusively involved therapeutic or physiotherapy-type exercise, such as targeted upper-limb rehabilitation for lymphoedema or stretching for cording; (v) prehabilitation studies where women received an intervention whilst awaiting primary treatment; (vi) interventions delivered exclusively at home or remotely; (vii) article has been retracted. We will identify retractions using the Retraction Watch Database (http://retractiondatabase.org/RetractionSearch.aspx) and using PubMed.
Study selection
After the literature searches are complete, citations will be collated and uploaded into a reference management tool (Endnote version 20, Clarivate Analytics, PA, USA). Following the removal of duplicates, the remaining studies will be uploaded into a web-based systematic review management tool Rayyan Systems Inc., Cambridge, MA, USA) to identify potentially eligible studies. Two reviewers will independently screen the titles and abstracts against the eligibility criteria. Full texts will be obtained for all studies that appear relevant or where there is any uncertainty. The same two reviewers will then independently examine each full-text manuscript against the eligibility criteria. Reasons for excluding full-text manuscripts will be recorded and reported in a PRISMA-ScR flow diagram57. Any disagreements between reviewers will be resolved through discussion and, if needed, consultation with a third reviewer. If it is necessary to clarify aspects of the study in relation to the eligibility criteria or retrieve a full-text manuscript, we will contact corresponding authors via email on at least two occasions within a one-month period. We will report how many authors were contacted and, of those, how many authors responded and sent data/information. We will share the full search and selection history on OSF (https://osf.io/xver2/).
Data extraction
Data will be extracted onto project-specific data extraction forms (DEFs) in Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA). DEFs will first be piloted; two reviewers will extract data independently from two studies and compare results. The data extraction forms will then be modified and revised if necessary. One reviewer will extract data from the remaining studies, which will be cross-checked in full by a second reviewer on completion. Discrepancies in data extraction between reviewers will be resolved through discussion and then, if needed, with input from a third reviewer. Any modifications to the data extraction forms will be detailed in the final report.
Data items extracted onto the primary DEF will include details on: title, authors, year of intervention implementation, year of publication, journal, country, study design, recruitment setting and method, eligibility criteria, participant characteristics, sample size, and reported outcomes including information on recruitment, retention, adherence, and adverse events. A secondary DEF will be used to capture data items related to the intervention development process (e.g., use of published intervention development approaches, if/how stakeholders contributed to the development process) and intervention characteristics (e.g., intervention rationale, theoretical basis, content, any concomitant interventions). Parameters for this DEF will draw on the Guidance for Reporting Intervention Development Studies in Health Research (GUIDED)58 and the Template for Intervention Description and Replication (TIDieR)59 checklists. If a manuscript cites a published protocol, manuscript, or a trial registration where details of the intervention are described in more detail, we will extract relevant information from these sources.
Data analysis and presentation
Extracted data will be narratively synthesized in tabular and text form. Where relevant, basic frequencies and percentages will be used to describe characteristics of the included studies. A narrative summary of the data will describe how the results relate to the review’s objectives.
Study status
As of 22 October 2024, we have registered the protocol, completed the searches, and screened studies for eligibility. Data extraction has not yet been undertaken.
Aim
To explore barriers and facilitators to physical activity in breast cancer care from different stakeholder perspectives.
Objectives
To explore and understand:
i. Women’s views and experiences of, and barriers and facilitators to, physical activity after finishing treatment for primary breast cancer.
ii. HCP’s views on organisational- and individual-level barriers to offering physical activity support in breast cancer care, and potential opportunities for offering referrals to community-based physical activity programmes.
iii. Exercise professional's views and experiences of delivering physical activity programmes for people with cancer, and special physical activity considerations for women after breast cancer treatment.
Study design
We will undertake qualitative, in-depth focus groups (supplemented with one-to-one semi-structured interviews, depending on preference) with three stakeholder groups: (i) women who have finished treatment for primary breast cancer; (ii) HCPs involved in breast cancer care; and (iii) exercise professionals. Findings will be reported in line with Standards for Reporting Qualitative Research (SRQR) guidelines60.
Study setting
We will recruit participants from across the UK through a variety of routes (see Recruitment and consent process). Newcastle University will sponsor this study. This study was approved on 12 December 2023 by the Faculty of Medical Sciences Research Ethics Committee, part of Newcastle University's Research Ethics Committee (ref: 39270/2023). Written informed consent will be obtained from all participants before data collection.
Participant eligibility
Eligibility criteria are presented in Table 1.
Sampling
Maximum variation sampling61 will be used to capture a wide range of perspectives and experiences. For women with breast cancer, we will aim to maximise diversity of age, socioeconomic status, ethnic background, and treatment received. For HCPs and exercise professionals, we will seek to maximise diversity based on professional role (e.g., nurse, surgeon, oncologist), years of professional experience, and geographical location. We will also seek to recruit exercise professionals working across different sectors.
Sample size
The final sample size will be guided by conceptual depth, which is a pragmatic decision based on the adequacy of the data (e.g., its richness, complexity, diversity)62. Based on recent sample size recommendations for qualitative research with homogenous populations and well-defined objectives63,64, we anticipate that we may need to hold 4–8 focus groups with each stakeholder group, with each focus group involving approximately 4–6 participants, to generate adequate data. Most published guidance on sample sizes for qualitative research focus on data saturation63; however, this concept has received criticism and is considered to be incompatible with the values and assumptions of reflexive thematic analysis, which does not consider codes to be fixed65.
Recruitment and consent process
We will use multiple strategies to recruit women, HCPs, and exercise professionals, including sharing study information through: (i) social media platforms; (ii) Breast Cancer Voices’ monthly bulletin (breastcancernow.org/get-involved/volunteer-us/breast-cancer-voices); (iii) breast cancer support groups and PPI groups; (iv) snowballing within our existing clinical, academic, and patient networks; and (v) community leaders, established charities, and professional organisations. To maximise the diversity of women, we will: (i) specifically encourage and target women from minority ethnic backgrounds to participate through our study adverts; (ii) target support groups in areas of high socioeconomic and ethnic diversity; (iii) approach leaders of underrepresented communities, including those serving South Asian, Muslim, and other minority women.
Potential participants will be given access to an online form (Microsoft Forms, Microsoft Corporation, Washington, USA; https://forms.office.com) and asked to express their interest by providing their name, contact details, and permission to be contacted. A research team member will email interested participants a separate online form containing the full eligibility criteria, participant information sheet (PIS), consent form, and sociodemographic questionnaire, and request details of their time availability and data collection preferences (i.e., focus group or interview; online or in-person). After the participant has provided written informed consent and submitted the form, a research team member will email them an invitation to a focus group or interview. Participants will have the option of receiving the study documents via post instead and returning them in pre-paid envelopes. To identify and prevent potentially fraudulent participation, we will remain vigilant to ‘red flags’66 and check photo ID before the start of focus groups.
Data collection
Sociodemographic details collected will include age, ethnicity, sex, postcode, highest level of education, employment status, date of diagnosis, tumour stage and hormone receptor status, treatment received, medial comorbidities, and disability status. For HCPs and exercise professionals, we will collect information on age, professional role, qualifications, years of experience, current employment status, and employer.
The default will be to conduct focus groups online with videoconferencing platforms, using either Zoom (https://www.zoom.com) or Microsoft Teams (https://www.microsoft.com/en-gb/microsoft-teams/group-chat-software). However, we will also consider holding focus groups in-person if enough participants within the same geographical area express as a preference for this approach. We will also offer the option of one-to-one semi-structured interviews held online, via telephone, or in-person in a public space of the interviewee’s choice (e.g., University campus, community centre). Offering multiple options for participation in qualitative research is an evidence-based strategy to increase participation in marginalised groups67.
At the beginning of the focus group, the interviewer will spend time building rapport with participants to establish a secure and trusting environment68. The interviewer will use a conversational-style approach whilst referring to stakeholder-specific topic guides to guide discussions, which will be informed by existing evidence69–73 and the COM-B model51. Additionally, the topic guide for HCPs will be informed by Normalisation Process Theory (NPT)74 to encourage participants to consider processes that could influence implementation. Topic guides will be used flexibly to allow participants to raise additional topics which they consider important. Participants will be encouraged to talk freely about their experiences and views, and topics will be discussed in the order that they arise naturally. Topic guides will evolve iteratively over the course of the study to allow any new issues raised to be explored in subsequent focus groups/interviews. Final topic guides will be made available on OSF.
Focus groups are expected to last approximately 90-minutes and interviews are expected to last approximately 60-minutes (this will be guided by how much participants want to contribute). Online focus groups will be audio-recorded through in-built recording features in videoconferencing software. Focus groups conducted in-person or via telephone will be audio-recorded using a digital voice recorder. Recordings will be transcribed verbatim by a Newcastle University-approved transcription service.
Data analysis
Anonymised transcripts from focus groups/interviews with the three stakeholder groups will be analysed separately using reflexive thematic analysis from a post-positivist epistemological perspective. Reflexive thematic analysis is compatible with the epistemological positioning of the research team and is able to capture latent and semantic meaning within the dataset75. Analysis will occur concurrently with data collection to ensure any new issues raised are explored in subsequent interviews76. Two research team members will work collaboratively to inductively identify and refine codes within each dataset and engage in constructive and reflexive dialogue (e.g., challenging assumptions) to construct initial themes. Final themes and subthemes for each stakeholder group will be agreed by the research team. Sub-themes relating to women’s barriers to, and facilitators, of physical activity, and HCP’s/exercise professional’s barriers and facilitators of providing physical activity support to women with breast cancer, will be mapped onto the COM-B model51. A phase of triangulation will then compare and contrast the themes/sub-themes across datasets to develop a comprehensive understanding of the barriers and facilitators to physical activity in breast cancer care, identify potential implementation problems, and inform intervention development. The Behaviour Change Wheel51 will be used to guide the selection of intervention functions, and the BCT Ontology77 used to identify appropriate BCTs to support women’s attendance at the community-based physical activity programmes and subsequent maintenance of physical activity. Analysis will be facilitated by NVivo software78.
Study status
As of 22 October 2024, we have completed 10 focus groups and one interview with 35 women in total, with qualitative analysis of anonymised transcripts being undertaken. We have held one focus group and 15 interviews with 19 healthcare professionals, with more interviews scheduled and qualitative analysis ongoing. We have not yet undertaken any qualitative research with exercise professionals.
Aim
To co-develop, operationalise, pre-test, and refine components of the PURE-EX programme.
Objectives
To co-develop:
i. A prototype referral pathway, enabling HCPs to refer women to community-based physical activity programmes after they have finished primary treatment for breast cancer.
ii. A prototype online training course to train community providers how to adapt their existing physical activity programmes for women who have had breast cancer treatment.
Design
We will hold a series of co-development events, using a sequential and systematic co-design approach79, to collaboratively design a prototype referral pathway and online training course.
Co-development workshops
We will form a Clinical Advisory Group (CAG) comprising 6–8 HCPs involved in breast cancer care, a Physical Activity Advisory Group (PAAG) comprising 4–6 exercise professionals with experience of delivering physical activity programmes to people with cancer, and a User Advisory Group (UAG) including 12–15 women who had finished treatment for early-stage or locally-advanced breast cancer. CAG and PAAG members will be recruited through existing clinical and professional networks and new networks developed in WP2. We will aim to maximise diversity of CAG members in terms of professional role (e.g., clinical nurse specialist, oncologist, surgeon) and geographical location. We will recruit UAG members through various sources, including through our PPI panel and breast cancer support groups, as well as approaching women who registered an interest or took part in our focus groups in WP2, aiming to maximise diversity of member’s age, socioeconomic status, and ethnic background. We will work with the CAG, PAAG, and UAG, in a series of in-person and online workshops (60–90-minutes each), to co-develop, operationalise, and refine programme components. Workshops will be facilitated by an experienced co-design researcher, assisted by at least one other member of the research team, who will take notes. We will present initial ideas about programme components in the workshops, including the referral process and features of physical activity programmes for women after breast cancer treatment. These initial ideas will be informed by a range of evidence sources, including physical activity guidelines for cancer survivors19, the Moving Through Cancer Initiative34,35 3A’s approach36, findings from the scoping review (WP1) and qualitative research (WP2), expert knowledge, NICE recommendations on exercise referral schemes38, and through discussions with our PPI panel. Refinements will be made in an iterative manner, with feedback obtained on the initial ideas, refinements made, and refined programme components being re-presented in the subsequent workshops until consensus is reached. We have previously used this approach successfully to co-develop multi-component interventions for women with breast cancer80,81. We will hold a final ‘consensus’ workshop with all stakeholder groups, integrating findings from WP1, WP2, and the workshops, to present and consolidate features of the programme.
Pre-testing
We will pre-test and refine programme components before the feasibility trial. First, we will observe HCPs (n=2–3) offering a physical activity referral to a PPI panel member. Observations will provide information on how the benefits of physical activity are communicated, the acceptability of enacting the referral, and immediate perspectives of HCPs delivering, and PPI panel member receiving, the referral. Second, exercise instructors (n=2–3) will complete the online training course, and through brief discussions held remotely via telephone or videoconferencing (depending on preference), we will gather their feedback on its content and coherence. Third, after completing the training course, instructors will deliver a physical activity session to our PPI panel members (n=10). We will explore immediate perspectives of instructors delivering the session, and women who took part, through brief surveys, comprising Likert-like and free-text items. Two to three days later, through brief discussions held remotely via telephone, videoconferencing, or asynchronous platforms (e.g., WhatsApp), we will ask instructors and PPI panel members to reflect on their experiences of the physical activity session. Following pre-testing and feedback, we will make refinements, finalise programme components (described using the TIDieR checklist59), document BCTs in each component (using the BCT Ontology77), and refine the logic model, if needed.
Study status
We are currently working with our PPI panel to co-develop workshop activities and materials. The workshops have not yet been undertaken.
Aim
Explore the feasibility and acceptability of the PURE-EX programme from different user perspectives.
Objectives
To assess:
i. The feasibility and acceptability of being offered referral to, and attending, a community-based physical activity programme in women who have finished primary treatment for breast cancer.
ii. The feasibility and acceptability of breast cancer HCPs offering physical activity referrals.
iii. The feasibility, acceptability, and views of instructors delivering a community-based physical activity programme to women after breast cancer treatment.
iv. The feasibility of data collection and potential signals of intervention efficacy.
Study design
This is a prospective, single-arm, pretest-posttest feasibility trial with integrated process evaluation. Trial processes, including recruitment procedures, eligibility criteria, and outcomes, will be collaboratively developed and agreed with our PPI panel. Following co-development of the PURE-EX programme and trial processes, a detailed protocol will be prospectively registered on a public registry before the first participant is recruited. A potential study schedule is presented in Table 2. Trial findings will be reported in line with the Consolidated Standards of Reporting Trials (CONSORT) extension to randomised pilot and feasibility trials82.
6MWT = Six-minute walk test; AE = adverse events; FACIT-Fatigue = Functional Assessment of Chronic Illness Therapy-Fatigue; FACT-B = Functional Assessment of Cancer Therapy-Breast; DASS21 = Depression, Anxiety and Stress Scale - 21 Items; HPLP-II = Health-Promoting Lifestyle Profile II; IPAQ-SF = International Physical Activity Questionnaire - Short Form; UKCC = UK Cancer Costs Questionnaire.
Study setting
Participants will be recruited from outpatient breast cancer clinics at Newcastle Hospitals NHS Foundation Trust and Gateshead Health NHS Foundation Trust, UK. Newcastle University will sponsor the trial. We will seek Health Research Authority (HRA) approval, including ethical approval from an independent NHS Research Ethics Committee. Written informed consent will be obtained from all participants before data collection.
Participant eligibility
Inclusion criteria
Women aged ≥18 years old.
Clinical diagnosis of early-stage or locally advanced breast cancer (stages I–III).
Finished primary hospital-based treatment for breast cancer (surgery, chemotherapy, and/or radiotherapy). Women receiving adjuvant endocrine therapy, targeted therapy, or immunotherapy will be eligible for inclusion.
Willing and able to give written informed consent.
Exclusion criteria
Diagnosed with metastatic breast cancer (stage IV).
Awaiting or currently receiving primary treatment for breast cancer (surgery, chemotherapy, or radiotherapy).
Uncontrolled cardiovascular or metabolic disease.
Pregnant or planned pregnancy within the study period.
Musculoskeletal, neurological, or rheumatoid condition that could be exacerbated due to participation in a physical activity programme, as determined by the referring HCP.
Sample size
There are no clear guidelines on sample size requirements for non-randomised feasibility trials. Our aim is to recruit 45 women into the study. Assuming a conservative loss to follow-up of 20%83, the research team considers this number of participants sufficient to achieve the study objectives and evidence suggests it is likely to achieve adequate qualitative data in post-study interviews64.
Participant identification and consent
A research nurse will identify potential participants via review of medical records ahead of routine follow-up appointments at the outpatient breast cancer clinic. Eligible participants will be sent an information sheet before their appointment and, if the participant is interested in taking part, written informed consent will be obtained at the clinic.
PURE-EX programme
The PURE-EX programme has two components: (i) a referral pathway, and (ii) an online training course for community providers. Research team members will train HCPs at recruiting sites to offer and enact referrals into community-based physical activity programmes. For this feasibility study, the physical activity programme will be delivered by a community health charity based in North East England (Healthworks, https://www.healthworksnewcastle.org.uk/). Healthworks employs qualified exercise instructors (Register of Exercise Professionals, Level 3 or 4) to deliver a range of physical activity programmes in community-based leisure centres, including for people with long-term health conditions, older people, and women. Healthworks-employed instructors will undertake the PURE-EX online training course to adapt their existing physical activity programmes for women with breast cancer (Figure 3).
Baseline data collection
Prior to participating in the physical activity programme, an instructor (who will be delivering the programme) will collect baseline information from each participant, including height, weight, blood pressure, sociodemographics (age, ethnicity, sex, postcode, highest level of education, employment status), and medical history (comorbidities, disability status, musculoskeletal injuries). Clinical information will be extracted from medical records by a research nurse, including date of cancer diagnosis, tumour grade, stage and receptor status, and treatment received.
Outcomes and mediators
The primary outcome is the feasibility of the PURE-EX programme. Cardiorespiratory fitness, physical function, and muscle strength will be assessed by the instructor before and after the physical activity intervention. Paper copies of study questionnaires and an accelerometer (for measurement of physical activity) will be given to participants to take home and return by post in pre-paid envelopes, or participants will complete study questionnaires online (Microsoft Forms), depending on preference.
Feasibility
We will document recruitment rates, withdrawals (with reasons), and loss to follow-up. We will record the proportion of participants who accept the physical activity referral and attend the community-based physical activity programme. A research team member attending a random sample (≈20%) of physical activity sessions will assess fidelity to the delivery of the physical activity programme using a standardised checklist, informed by TIDieR59. Reporting of adverse events will be conducted in line with the study sponsor’s policy on adverse event reporting for non-clinical trials of investigational medicinal products; we will record all serious adverse events, as well as all non-serious adverse events that are deemed to be related to study participation.
Acceptability
Acceptability of PURE-EX programme components will be assessed using a validated acceptability questionnaire based on the theoretical framework of acceptability (TFA)84. The questionnaire consists of 9 items: one item reflecting each of the TFA constructs of affective attitude, burden, perceived effectiveness, intervention coherence, self-efficacy, and opportunity costs, two items reflecting ethicality, and one general acceptability item.
Patient-Reported Outcomes (PROs)
The Functional Assessment of Cancer Therapy-Breast (FACT-B) total score will be used to measure disease-specific QoL85. Fatigue will be measured with the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) total score86. Anxiety and depression symptoms will be assessed via the Depression, Anxiety and Stress Scale - 21 Items (DASS-21) total subscale scores87. We will administer the EQ-5D-5L88 and a modified version of the UK Cancer Costs Questionnaire (UKCC) to assess the feasibility of collecting health economics data.
Cardiorespiratory fitness
The six-minute walk test (6MWT) is reliable (intraclass correlation coefficient = 0.93) and correlates well (r = 0.67) with peak oxygen consumption achieved during a cardiopulmonary exercise test89. Participants will be instructed to walk at a self-selected maximal pace back and forth along a flat surface, covering as much distance as they can in six minutes. All instructions, encouragement, and monitoring will adhere to American Thoracic Society Guidelines90. At the end of the test, participants will be asked to stop where they are and the total distance covered will be measured in meters.
Physical function
The Short Physical Performance Battery (SPPB), a composite measure of standing balance, five chair stands, and 4-meter gait speed, will be used to assess physical function. Each test is assigned a categorical score from 0 (worst performance) to 4 (best performance) according to standardised criteria. A total score from 0 to 12 is obtained by summing the scores from the three tests.
Five chair stands. Participants will sit in a firm, armless chair with their arms crossed over their chest. They will position themselves at the edge of the seat to reduce trunk flexion91 and be instructed to stand fully upright (legs straight) and then return to a seated position (full weight on the chair) five times as quickly as possible, while maintaining proper form. A practice repetition will ensure correct technique, followed by one timed trial. The completion time for five sit-to-stands will be recorded in seconds.
Standing balance. Participants will stand unassisted with their feet placed in three different positions: side by side, semi-tandem, and tandem, each for up to 10 seconds. Timing will stop either at 10 seconds or when the participant loses balance (e.g., stepping out of position or grabbing support). One trial will be conducted for each stance, and the duration will be recorded in seconds.
4-meter gait speed. Participants will walk 4 meters at their usual pace, with the time recorded in seconds. Walking aids will be allowed if required.
Hand grip strength
Participants will use their dominant hand to squeeze a hand-held digital grip dynamometer as hard as possible for 3 seconds. They will remain seated with their arm fully extended by their side during the test. The maximum score from three trials will be recorded in kilograms.
Mediators
Device-based physical activity will be measured using a tri-axial accelerometer (Axivity AX3, Axivity Ltd, Newcastle upon Tyne, UK)92 worn on the non-dominant wrist for 7 consecutive days, which is a well-established proxy for habitual physical activity93. Data will be considered valid if participants wear the accelerometer for at least 8 hours on at least 4 days94. From the raw accelerometer data, we use the GGIR package95 in the latest version of R (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/) to derive time spent sedentary and time spent in light, moderate, and vigorous activity. Published cut-points based on metabolic equivalents (METs) will be used to demarcate the intensity of physical activity96. We will also assess self-reported physical activity using the International Physical Activity Questionnaire – Short Form (IPAQ-SF)97, which provides data on time spent sitting and MET-minutes per week of walking and doing moderate and vigorous activity. The Health-Promoting Lifestyle Profile-II (HPLP-II) total score will be used to assess the extent to which participants engage in health-promoting behaviours. The HPLP-II comprises 52 items that measure six dimensions of a health-promoting lifestyle: health responsibility, physical activity, nutrition, spiritual growth, interpersonal relations, and stress management.
Qualitative process evaluation
We will invite participants to take part in semi-structured interviews at two timepoints: (i) 1–2 weeks after receiving the referral; and (ii) 1–2 weeks after completing the physical activity programme. Interviews conducted after the referral will explore experiences of interactions with HCPs, the referral pathway, and barriers and facilitators to accepting the referral and accessing the physical activity programme. Interviews held after programme completion will explore experiences of the physical activity programme and barriers and facilitators to sustained adherence. The interview topic guide will be informed by COM-B and our findings from the qualitative research in WP2. We will interview HCPs (n≈4-6) to explore experiences of the carrying out the referral and how it fitted into routine care, with the topic guide structured around COM-B and the constructs of NPT. Additionally, we will interview instructors to explore views of the online training course and barriers and facilitators to delivering the physical activity programme. Interviews will last approximately 60-minutes and be conducted in-person or remotely via telephone or videoconferencing (depending on interviewee preference), audio-recorded, and transcribed verbatim.
Qualitative analysis
Anonymised transcripts will be analysed, concurrently with data collection76, with thematic analysis using a combined deductive and inductive approach98. Transcripts will be coded deductively line by line using a pre-established coding scheme that aligns with the research questions and is guided by process evaluation recommendations99. Following coding completion, each section of the coding scheme will be subject to an inductive thematic analysis, in which salient themes and recurrent patterns within the data will be identified and sub-themes created within each section100. Data will be mapped onto the COM-B model and TDF to identify mechanisms through which the intervention brought about change51,52. NPT will also be used to structure the coding framework to identify how new behaviours and practices can be integrated within routine clinical care74. Analysis will be facilitated by NVivo software78.
Quantitative analysis
The flow of patients throughout the trial will be reported in a CONSORT flowchart82. Descriptive statistics will be used to present baseline characteristics and feasibility outcomes. A paired t-test or Wilcoxon signed-rank test (depending on data distribution, assessed via visual inspection of histograms and Q-Q plots) will be used to evaluate changes in outcomes from baseline to post-intervention, with the mean change and 95% confidence interval presented101. Participants with missing data will be excluded from the analysis. The analysis will be performed in the latest version of R.
Study status
We are currently co-developing the detailed trial protocol with our PPI panel.
Survivors of breast cancer often face long-term physical and mental health challenges, including cancer-related fatigue, reduced QoL, and increased risk of chronic health conditions5,6,8,15. Evidence-based guidelines recommend HCPs advise women to engage in regular physical activity to mitigate the adverse effects of breast cancer and its treatment17–19. However, support to be active is not standard practice. The PURE-EX programme aims to integrate physical activity referrals into standard care for women after breast cancer treatment by establishing a new referral pathway and co-developing an online training course to train community providers on how to adapt their existing physical activity programmes for women with breast cancer.
HCPs report many barriers to providing physical activity support to women with breast cancer. Organisational barriers are central to most issues32 and include limited resources, absence of a referral pathway, and a lack of suitable physical activity programmes to refer to29–32. Recent initiatives have recommended HCPs refer patients without contraindications to community-based physical activity programmes34,35. Physical activity programmes are widely accessible across diverse communities, and therefore leveraging the existing community infrastructure could offer a solution to improve physical activity support in breast cancer care. However, generic physical activity programmes do not accommodate for the unique physical and psychosocial needs of women who have undergone breast cancer treatment, and are thus less likely to be acceptable or effective. For example, offering breast cancer survivors referral to a community leisure centre or cardiac rehabilitation programme, without any modifications to address breast cancer-specific determinants of physical activity, led to poor uptake and engagement102. We propose that upskilling existing community providers on how to adapt their physical activity programmes for women with breast cancer may be an efficient and scalable approach to make physical activity support routinely available.
The feasibility trial will address key uncertainties relating to feasibility and acceptability of the PURE-EX programme, including whether HCPs find it acceptable to offer and enact physical activity referrals, whether women accept the referral and attend the physical activity programme, and whether it is feasible for instructors to effectively adapt their existing programmes. Following trial completion, we will refine the intervention and logic model to maximise feasibility and the likelihood of effectiveness. The final output will be an evidence-based, theory-informed, person-centred intervention that is feasible and acceptable to women, HCPs, and instructors.
The safety and efficacy of physical activity for people with cancer is well-established. However, there is limited knowledge on its clinical effectiveness in “real world” settings and about how to integrate physical activity into routine cancer care. This knowledge gap is evident in clinical practice, as only one in five women with breast cancer recall receiving physical activity advice after completing treatment23. Therefore, a subsequent hybrid effectiveness-implementation trial design could provide the necessary evidence to support the implementation the PURE-EX programme into routine care. If we find the programme to be feasible and acceptable, the next steps to wide-scale implementation will be determined by our research team, based on the findings, funding landscape, and guidelines for evaluating complex interventions49.
Ethical approval for the qualitative research (WP2) and co-development workshops (WP3) was granted on 12 December 2023 by the Faculty of Medical Sciences Research Ethics Committee, part of Newcastle University's Research Ethics Committee (ref: 39270/2023). For the feasibility trial (WP4), we will seek Health Research Authority (HRA) approval, including ethical clearance from an independent NHS Research Ethics Committee. Written informed consent will be obtained from all participants prior to data collection.
No data are associated with this article.
Anonymised quantitative data will be made publicly available on the Open Science Framework within one month of publication in a peer-reviewed journal. Anonymised transcripts will be available upon reasonable request from the corresponding author.
No extended data are associated with this article.
Extended data will be made publicly available on the Open Science Framework within one month of publication in a peer-reviewed journal.
WP1: The systematic scoping review will be reported following the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR)57.
WP2: Findings from the qualitative research will be reported in accordance with the Standards for Reporting Qualitative Research (SRQR) guidelines60.
WP3: As no established guidelines exist for reporting co-development workshops, we will draw on the team's extensive experience in conducting and reporting such activities to ensure transparency and replicability.
WP4: Trial findings will be reported following the Consolidated Standards of Reporting Trials (CONSORT) extension for randomised pilot and feasibility trials82.
PPI: Patient and public involvement (PPI) activities will be reported using the ‘Guidance for Reporting Involvement of Patients and the Public, Version 2’ (GRIPP2) checklist103.
Ms Rosie Glossop (Newcastle University) contributed to the design of the systematic scoping review and conducted the database searches.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)