Keywords
Systematic review; Sunlight; Ultraviolet radiation; Mortality; Cancer; Cardiovascular disease
Current sun safety advice focuses on minimizing exposure to sunlight, due to the relationship between ultraviolet radiation and skin cancer. However, sunlight also has beneficial effects, and there are calls for guidance to reflect these alongside the harmful effects. To examine the net effect of harmful and beneficial aspects, we aimed to determine the association between sunlight exposure and all-cause mortality. Additionally, we examined cause-specific mortality and whether the associations varied according to skin type/colour or ethnicity.
We conducted a systematic review, searching MEDLINE, Embase, Web of Science and the Cochrane Central Register of Controlled Trials (Nov 2023) for reports of epidemiological studies in the general population investigating the effect of long-term sun exposure on all-cause, cardiovascular-related, or cancer-related mortality. We conducted a narrative synthesis of the findings and assessed risk of bias using the ROBINS-E tool. PROSPERO: CRD42023474157.
The search identified 73 eligible articles, with 55 included in the narrative synthesis. Methods of measuring sunlight exposure comprised radiation, proxy measures of radiation (e.g., latitude) and behaviour associated with sunlight exposure. The evidence was mixed. While most studies of skin cancer mortality found a higher risk associated with more exposure to sunlight, many studies of other cancers reported lower associated risk. Evidence for all-cause mortality was mixed, as were findings for cardiovascular mortality. Results were subject to high risk of bias, largely due to the likelihood of uncontrolled confounding and the use of indirect measures of sunlight exposure. There were insufficient data regarding any differential effects of sunlight on mortality for those of different skin types/colours or ethnicity.
Findings from observational epidemiological studies of the association between sunlight exposure and mortality vary across different disease outcome and location being investigated. As such, the findings do not provide a strong rationale for changes to sun protection guidance.
The effect of sunlight on death rate: a systematic review of the evidence
Sunlight exposure of the skin can lead to the development of skin cancer. Therefore, sun safety advice in the UK is mainly focused on avoiding the harmful impacts of sunlight. Organizations providing advice on sun exposure say to wear sunscreen and covering clothing, and to stay in the shade between 11 am and 3 pm.
There are also positive effects of sunlight exposure such as vitamin D production in your body. Vitamin D is important for your bones. Vitamin D or other effects of sunlight may also help reduce your risk of developing some cancers and cardiovascular disease.
People of different skin types react to sunlight in different ways. People with darker skin need more exposure to sunlight than people with lighter skin to produce the same amount of vitamin D, and they also have less risk of developing sun-related skin cancer.
We gathered all the available studies that have measured both sunlight exposure and mortality (death) rates. We were interested in the overall number of deaths (by any cause), as well as deaths specifically caused by cardiovascular disease or cancer.
Our findings are mixed. Exposure to sunlight has been reported both to increase and to decrease your risk of dying. Alongside its harmful effect on skin cancer, sunlight may help prevent other types of cancer. However, there were issues with the amount of data available, as well as the quality of some of the data that was available, so we can’t be certain about the findings. Currently, there is not strong enough evidence to alter sun exposure advice and so people should continue to follow the guidance.
Systematic review; Sunlight; Ultraviolet radiation; Mortality; Cancer; Cardiovascular disease
There have been minor changes to the wording of the abstract, for example to highlight that only 55 of the included articles were utilised in the narrative synthesis. There were also minor edits to the wording of the plain language summary, e.g., to include reference to the use of sunscreen in current guidance. The figures have all been amended in order that the x-axis now represents the exponential scale. Additionally, Figure 1 has been amended to show that 55 articles were included in the narrative synthesis. Minor changes were made to the Tables to correct issues with inconsistent footnoting and to include clearer description of the of the specific outcome for all regression/correlation results. In the methods section, we have expanded the detail on the ROBINS-E and GRADE tools. In the results section, we have provided more in-depth detail on the types of risk of bias issues commonly found across results, as well as providing clarity on the confounders we assessed and those which we commonly encountered across the studies. The bulk of the changes in this version occur in the discussion. Here we have added new discussion points around the potentially moderating effect of skin type on the relationship between exposure and geographic location, as well as a discussion around the difficulty of disentangling the effects of sunlight exposure on the skin from associated confounders and lifestyle factors. Additionally we have added a several suggestions for future directions for both primary research, as well as potential avenues to expand the utility of the data extracted in this review.
See the authors' detailed response to the review by Carolyn Heckman
See the authors' detailed response to the review by Richard McKenzie, Ben Liley and James Liley
See the authors' detailed response to the review by Leonardo Vinícius Monteiro de Assis
See the authors' detailed response to the review by Douglas Brash
See the authors' detailed response to the review by Rasha Shraim
Current sun safety advice is primarily focused on minimizing exposure to sunlight. For example, in the United Kingdom (UK) this includes Cancer Research UK recommendations to keep to the shade between 11 am and 3 pm and to cover up with clothes1, advice that is echoed by the National Health Service (NHS)2 and the National Institute for Health and Care Excellence (NICE)3, and in the USA by the National Cancer Institute (NCI)4. The reason behind this advice is the well-established relationship between exposure to ultraviolet (UV) radiation (UVR) and skin cancer5. It has been estimated that 86% of melanoma cases are attributable to UVR6, and the risk of developing basal cell carcinoma (BCC), the most common form of non-melanoma skin cancer (NMSC), is 1.86 and 2.12 times greater with every five sunburns experienced as a child and as an adult, respectively7. Cutaneous squamous cell carcinoma (cSCC), which is capable of metastasizing, is the other common type of NMSC, and shows an increasing incidence in the UK and various European countries8,9.
There are calls to alter sun exposure advice10. The harmful effects of sunlight exposure should be considered alongside any beneficial effects. For example, sunlight exposure is usually the body’s main source of vitamin D11, which is linked to various health benefits, such as reducing cancer risk and improving immune system functioning, in addition to its established benefit in musculoskeletal health12–14. Other mechanisms may also contribute to health. Evidence suggests that sunlight converts nitric oxide metabolites, stored in the skin, to nitric oxide15, which may help to reduce blood pressure, amongst other actions that may be beneficial to cardiovascular health16. Furthermore, sun protection advice often fails to consider different skin types fully. People of different skin types react to UVR in different ways, resulting in different needs. A recent review found little evidence of an association between melanoma incidence and UVR in people with skin of colour17, suggesting that sunlight exposure may not be a risk factor for melanoma in those with darker skin, as it is for those with lighter skin18.
Given the various risks and benefits associated with sunlight exposure, there is a need to examine the overall effect on population mortality to help people find the right balance between gaining the benefits of sunlight exposure whilst minimizing the risks. We sought to examine the global evidence on the association between sunlight exposure and mortality. Our main aims were to estimate the effect of exposure to sunlight on all-cause mortality, cardiovascular-related mortality and cancer-related mortality. We additionally examined evidence on whether the effects of exposure to sunlight on mortality vary according to skin type/colour or ethnicity. We undertook a systematic review following principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care19 and the Cochrane Handbook for Systematic Reviews of Interventions20.
We formed a public advisory group comprising four individuals with different skin types (three with dark skin and one with light skin). We obtained input into the protocol from this group, although their ability to influence it was limited since the project was commissioned. They explained that people with darker skin can be very concerned about the harms of sunlight exposure as well as the possibility of vitamin D deficiency, reinforcing the need to examine data by subgroups defined by skin colour and type. They also made it clear that more refined guidance would be useful for people with all types of skin. They strongly supported the project and advised us that the findings would be of great interest. They further advised us on how we might disseminate the findings to those responsible for national guidelines on sun exposure; they felt that their health providers (e.g. general practitioners) were not sufficiently informed to provide targeted advice. Members of the public helped us write a lay summary of our plans for our website in advance of the project start (https://bristol-esg.org/projects-2/sunlight-exposure/) and a member of the public advisory group helped us write a plain language summary to disseminate the results.
Criteria for inclusion of articles in the review were: (i) reporting a primary epidemiological study with a cohort design (including randomized trials of interventions to alter relevant behavioural exposures), a case-control design or an ecological design; (ii) in a general population; (iii) using any measure(s) of long-term sunlight exposure; and (iv) reporting outcome data on all-cause mortality, cancer-specific mortality or cardiovascular disease (CVD) mortality. To perform a comprehensive assessment, we used the exposure term ‘sunlight’ broadly to include measures of radiation (such as sunlight, UVR and UVB); proxies for radiation measures (such as latitude and geographical location); and behaviours associated with sun exposure (such as episodes of sunburn and recreational sun exposure). We excluded: (i) studies of artificial sources of UVR exposure, such as sunbed use and prescribed phototherapy and (ii) studies restricted to people with pre-existing disease (e.g. reporting survival rates in people diagnosed with melanoma).
We searched MEDLINE, Embase, Web of Science and the Cochrane Central Register of Controlled Trials to November 2023 using relevant controlled vocabularies, text-words and search syntax appropriate to each resource. See Appendix S1 in the extended data21 for the full search strategy. Additionally, we performed forwards and backwards citation searches of included articles identified in the search and scanned the reference lists of relevant systematic reviews. We also checked for any relevant retraction statements or errata of included studies. Two reviewers independently assessed all reports for eligibility.
Data were extracted using standardized data extraction forms developed in Microsoft Excel, which had first been piloted on a small sample of articles and adapted as necessary. We collected the following data: study design (nested case-control, case-control, cohort, ecological, trial, quasi-experimental), funding sources (public, industry, mixed), study location, sex, age, ethnicity/race, skin type/colour, occupation, inclusion criteria, method/definition of sunlight exposure, period of exposure (childhood, adolescence, adulthood), length of exposure (specific, lifetime) and target condition (all-cause, cancer, CVD). We extracted summary data relating to the association between sun exposure and mortality overall and for CVD and cancer-related causes. This was reported differently across the studies included in this review, and included odds ratios (OR), risk ratios (RR), hazard ratios (HR), regression coefficients, correlation coefficients and narrative reporting.
Our main outcomes were death from any cause (all-cause mortality), death due to any cancer (all-cancer mortality) and death due to any cardiovascular cause22 (all-CVD mortality). We also examined death due to skin cancer (melanoma and NMSC) and the five most common causes of cancer-related mortality in the UK according to Cancer Research UK23: breast, prostate, lung, bowel and pancreatic cancer; as well as specific causes of CVD.
We assessed risk of bias in results using the Risk Of Bias In Non-randomized Studies of Exposure (ROBINS-E) tool for observational studies24. ROBINS-E is a tool designed to evaluate the risk of bias in results of observational epidemiologic studies, covering aspects of design, conduct and reporting of the study. It assesses risk of bias across seven domains: confounding, measurement of the exposure, selection of participants, post-exposure interventions, missing data, measurement of the outcome and selection of the reported result. Risk-of-bias assessments were not carried out on articles that reported results as correlations, mortality rates or narratively because the ROBINS-E tool aims to assess risk of bias in estimates of causal effects. Two reviewers independently assessed all reports considered potentially relevant for inclusion, extracted descriptive data and results of included articles, and assessed the risk of bias in results. Any disagreements were resolved by consensus or discussion with a third reviewer.
Due to the diversity in the types and units of measurement of the sunlight exposure measures, we did not consider it appropriate to conduct meta-analyses. Instead, we produced a narrative synthesis of the findings for each mortality outcome and examined any comparisons across people of different skin type/colour or ethnicity.
Articles reporting results as a relative risk (e.g., RR, HR; or data sufficient to derive these) were grouped by type of exposure and presented in forest plots, with estimates inverted as appropriate to illustrate findings using consistent direction of effect. Where an article reported only separate risk estimates for males and females, we combined these using fixed-effect meta-analysis to provide a result that additionally controlled for sex/gender.
Where results were presented in forms other than relative risks for two or more studies, we listed these in tables. Where such results were reported only separately for males and females these were treated as one analysis result (to be consistent with the combined relative risk results), but were reported as separate results in the tables.
We address certainty in the evidence through consideration of each of the five GRADE domains in the discussion. The GRADE domains assess the risk of bias in the included studies (risk of bias); how consistent the results are within each outcome (inconsistency); how applicable the evidence is to the review question (indirectness); how precise the estimates are (imprecision); and the possibility of selective publication of results (publication bias).
The database searches identified 8501 records, and 5181 records were identified using other methods. After examination of potentially relevant full text articles, 73 articles25–97 met the criteria for inclusion in the review (Figure 1). For a comprehensive summary of the characteristics of these 73 articles, see Table S1 in the extended data21.
Where multiple articles had used the same cohort, same exposure type and reported the same outcome, we classed these as ‘overlapping’. In these cases, we chose a main article as the source of data. The selection of data was based on an algorithm that included the type of analysis performed (ratio data preferred to linear regression or correlation coefficients), adjustment within analysis (most relevant confounders controlled for preferred), population (whole population preferred to specific subgroups), follow-up time (longer preferred), number of units of observation (e.g. counties preferred to states) and number of participants. As the majority of the included studies were ecological, we applied the same process to national datasets. We considered articles using national mortality data for the same country and date range to be using the same cohort, and used the same selection algorithm to select a main article in such cases. Table S2 in the extended data21 provides a review of all overlapping studies.
After the selection of main articles, this resulted in 55 articles being included in the narrative synthesis. All subsequent analysis is conducted on these 55 articles. Eight of the articles reported data on all-cause mortality, eight on all-CVD mortality, and 17 on all-cancer mortality. Eleven described cohort studies, three case-control studies and 41 ecological studies. We did not identify any eligible randomized controlled trials.
Twenty-four (44%) of the articles were conducted in the USA, five were conducted worldwide (including between 34 and 172 countries), four in China, and three each in Japan, Spain and Sweden. Other articles were conducted in the USA and Canada, the UK, and Switzerland (all n = 2); as well as Australia, Chile, Europe-wide, France, Italy, New Zealand and Turkey (all n = 1).
We provide descriptions of the exposures in Table S3 in the extended data21. The majority of the articles (n = 42, 76%) measured a single type of exposure; ten (18%) measured two types of exposure, two (4%) measured three types of exposure and one measured four types of exposure. Thus, we reported details of 72 exposure types in total. Of those, 40 were categorized as measures of incident radiation (herein referred to as ‘radiation’), 17 were proxy measures of radiation and 15 were measures of behaviours associated with sunlight exposure. Radiation measures included solar radiation, UVR, UVA and UVB, DNA- and erythemal-weighted UVB and UVR, UV Index, solar incidence, insolation, irradiation, sunlight hours and sunshine. Among the proxy measures for radiation, 16 were latitude (e.g. residential at county or state level) and one was geographic location of deployment during war (tropical vs European). Measures of sunlight exposure-related behaviour included four of occupational exposure, four of recreational exposure and one of both occupational and recreational exposure combined; three used skin damage, two used NMSC mortality rates and one used melanoma mortality rates.
Of the 55 included main articles, 34 (62%) reported information on participants’ skin type/colour or ethnicity. Most were conducted in only White participants, whilst two only contributed data for Black participants. In six articles, the authors reported that skin type/colour or ethnicity was mixed. Of these, four included Black and White participants (although, where proportions were reported, the populations were predominately White). One included Black, White and Hispanic participants, and one included Black, White, Hispanic, Asian and Native American participants.
The majority of results that were assessed for risk of bias were judged to be of some concern or high risk of bias. There were no results we judged to be low risk of bias across all domains, whilst some were assessed to be at very high risk of bias. The most common reasons for potential bias were uncontrolled confounding and concerns over the measurement of exposure. Concerns in the latter of these focused on the lack of direct measurement of personal sunlight exposure, as is largely inevitable in large population-based studies, as well as the use of aggregated measures that may have misclassified individual exposure. In order to judge the risk of bias due to confounding, we prespecified a number of important confounding factors which should ideally have been controlled for, including age, sex/gender, ethnicity, smoking status, socioeconomic status, physical activity and altitude. No study included in the review controlled for all of these factors. The most commonly controlled for factors were age and sex/gender, followed by smoking. Looking at the primary outcomes, around half of the results controlled for race/ethnicity, though this was predominately achieved through restricting analysis to White participants. Physical activity was less well controlled for, whilst almost no studies controlled for altitude.
Below we provide our findings for our primary outcomes: all-cause, all-CVD and all-cancer mortality, both overall and by type of exposure. Subsequently, we provide a brief summary of the findings for our secondary, cause-specific mortality outcomes. We report the full risk-of-bias assessments, including justifications for judgments, in Table S4 in the extended data21.
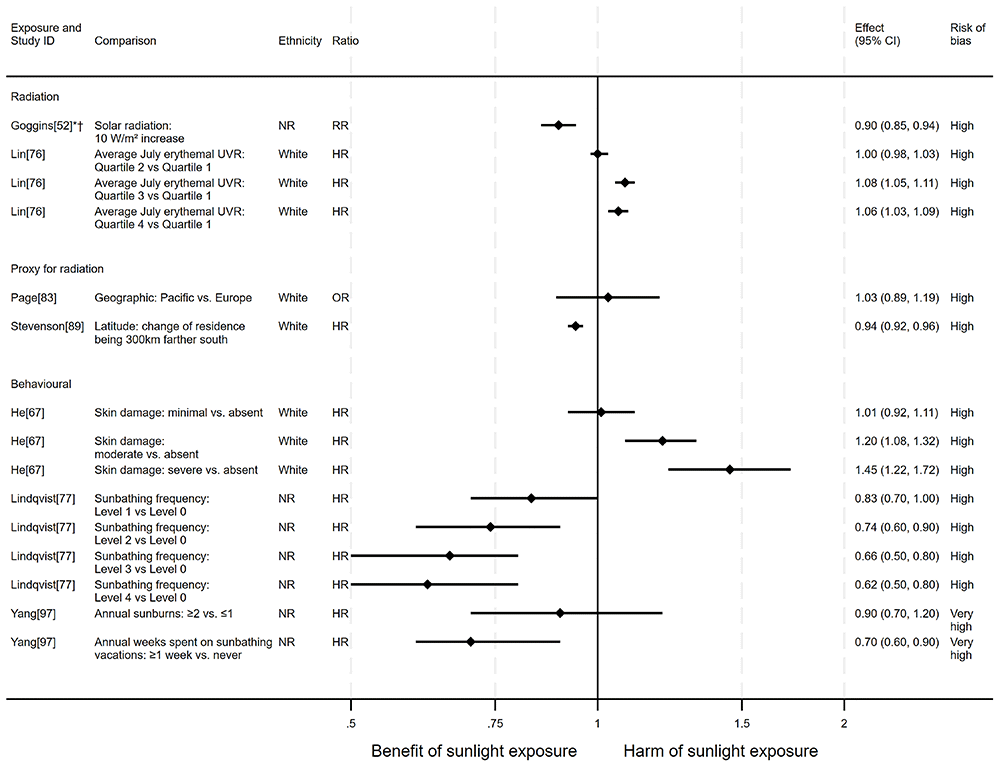
Overview. In total, eight articles investigated the effect of sunlight on all-cause mortality. Six used a cohort design and two had an ecological design. One article reported findings for two exposures (nine analyses reported across the eight articles). The findings were mixed (see Figure 2 for reported relative risks). Five analysis results52,77,89,97 were in the direction of a beneficial association between sunlight and all-cause mortality. Four analysis results47,67,76,83 were in the direction of a harmful effect of sunlight.
Radiation. Three articles looked at the effect of radiation on all-cause mortality (Figure 2). The results of all three articles were considered to be at high risk of bias. Goggins et al.52 conducted an ecological study of the Hong Kong population showing that a 10 W/m2 increase in solar radiation was associated with a 10% decrease in all-cause mortality (RR = 0.90, 95% confidence interval (CI) 0.85 to 0.94). Lin et al.76 measured the residential address of 346,615 cohort participants across six states and two metropolitan areas in the USA to determine average July erythemal UVR. This measure was subsequently split into quartiles. Their analysis included 41,425 participants who died from any cause. When comparing the second quartile with the first, there was no evidence of a difference in all-cause mortality (RR = 1.00, 95% CI 0.98 to 1.03; Figure 3). However, when comparing the third and fourth quartiles with the first there were 8% and 6% increases in all-cause mortality, respectively.
*Fixed-effects meta-analysis performed to combine gender subgroups. †Result inverted to reflect an increase in exposure. NR: ethnicity of population not reported.
In addition to the articles included in Figure 2, Fu and Wang47 examined the relationship between the average hours of daily sunshine in China and all-cause mortality using regression. They found that an increase of 0.1 to 0.2 in the average daily sunshine duration rate (equivalent to 2.9 hours of additional daily sunshine) was associated with an increase in all-cause mortality rate the following year (β = 11.509, 95% CI 1.87 to 21.15; high risk of bias).
Proxy for radiation. Two articles looked at the effect of proxy radiation exposures on all-cause mortality (Figure 2). The results of both articles were considered to be at high risk of bias. Stevenson et al.89 examined the effect of latitude in the UK using a cohort of 376,729 participants (27,111 all-cause deaths). They found that more southerly latitudes were associated with lower all-cause mortality, when compared with residences 300km further north (HR = 0.94, 95% CI 0.92 to 0.96). Page et al.83 examined a cohort of 9,237 WWII veterans (5,411 all-cause deaths) who were deployed to either Pacific or European battlefronts, arguing that those who were deployed to the Pacific area would have experienced higher levels of sun exposure than those in Europe. They observed a small increase in risk associated with being deployed in the Pacific compared with Europe, however the wide confidence intervals were compatible with both a benefit and harm (odds ratio = 1.03, 95% CI 0.89 to 1.19).
Behavioural. Three articles examined the effect of sunlight exposure behaviour (Figure 2). Heet al.67 looked at the effect of physician-assessed actinic skin damage among a cohort of 8,472 participants (2,969 all-cause deaths). The findings suggested that greater sun exposure, as indicated by skin damage, is associated with an increase in the risk of mortality. When comparing those with severe skin damage with those who were assessed to have no damage, there was a 45% increase in risk (HR = 1.45, 95% CI 1.22 to 1.72; high risk of bias). The association does not appear to be driven by smoking (an important common cause of skin damage and CVD), which was controlled for in the analysis.
Lindqvist et al.77 measured self-reported sun exposure in a cohort of 29,518 Swedish women (2,545 all-cause deaths). The evidence suggested that there may be a decrease in mortality with increased sun exposure. Those who reported the highest level of sun seeking behaviour had a 38% decreased risk of dying from any cause compared with those who reported the lowest level (HR = 0.62, 95% CI 0.50 to 0.80; high risk of bias). This finding is supported by Yang et al.97 in another cohort of 38,472 Swedish women (754 all-cause deaths), though their results were considered to be at very high risk of bias. Those who reported spending one week or more annually on sunbathing vacations had a 30% decreased risk of mortality compared with those who never went on sunbathing vacations (HR = 0.70, 95% CI 0.60 to 0.90). They also reported an association between higher number of sunburns experienced and lower all-cause mortality, however the wide confidence intervals were compatible with both a benefit and harm (HR = 0.90, 95% CI 0.70 to 1.20).
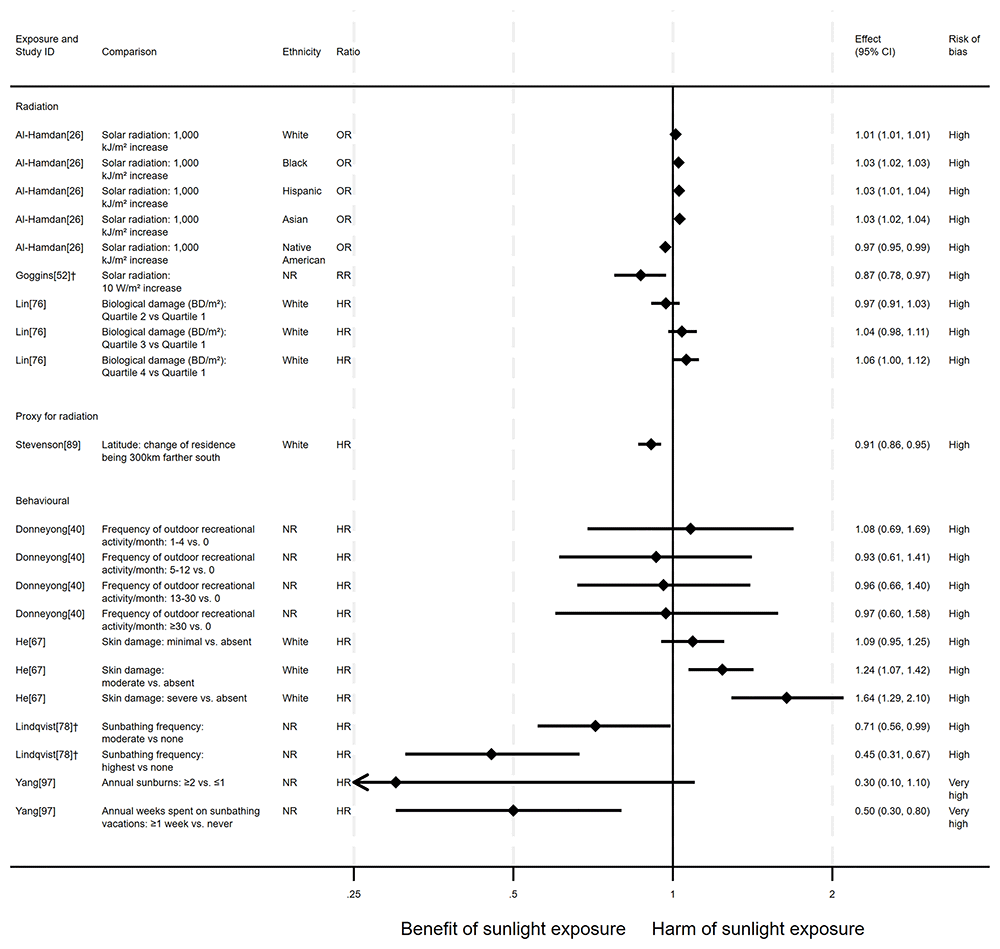
Overview. In total, eight articles investigated the effect of sunlight on overall CVD mortality, with one reporting findings for two exposure types (thirteen analyses reported across the eight articles). Six of the articles had a cohort design and two were ecological. There were mixed findings (Figure 3). Six analysis results26,52,78,89,97 suggested that higher levels of sunlight are associated with lower risk of CVD mortality. In contrast, five analyses26,67 suggested that higher levels of sunlight are associated with a higher risk of CVD mortality. Two analyses40,76 produced mixed findings.
†Result inverted in order to reflect an increase in exposure. NR: ethnicity of population not reported.
Radiation. Three articles looked at the effect of radiation, all were considered to be at high risk of bias (Figure 3). Al-Hamdan et al.26 performed a national ecological study in the USA. They found that a 1,000 kJ/m2 increase in solar radiation was associated with a 1% higher risk of dying from CVD for White participants, a 3% higher risk for Black, Hispanic and Asian participants, but a 3% lower risk for Native American participants. Goggins et al.’s52 Hong Kong-based study found that an increase in solar radiation of 10 W/m2 was associated with a lower risk of dying from CVD (RR = 0.87, 95% CI 0.78 to 0.97). In Lin et al.’s76 dose response analysis (n = 346,615; CVD deaths = 8,854) of average July residential erythemal UVR exposure, there were mixed findings. Risk of CVD mortality increased with dose. However, across all levels of comparison, the confidence intervals were compatible with both a benefit and harm, or with a null effect.
Proxy for radiation. Stevenson et al.89 found evidence to suggest a beneficial effect of sunlight exposure, measured via latitude in the UK (n = 376,729; CVD deaths = 5,389). Compared with northerly latitudes, residences 300 km further south were associated with a 9% lower risk of CVD mortality (HR = 0.91, 95% CI 0.86 to 0.95; high risk of bias).
Behavioural. Four articles examined the effect of sunlight exposure behaviour (Figure 3). Lindqvist’s et al.78 Swedish cohort study (n = 29,518) found that those who had self-reported high recreational sun exposure were less at risk of dying from CVD than those who reported no exposure (HR = 0.45, 95% CI 0.31 to 0.67; high risk of bias).
Yang et al.97 (n = 38,472; CVD deaths = 100) found that people who reported spending one or more weeks a year on sunbathing vacations were half as likely to die from CVD than those who never spent time on sunbathing vacations (HR = 0.50, 95% CI 0.30 to 0.80). Additionally, there was an association between higher frequency of sunburning and lower CVD mortality, however the wide confidence intervals indicate uncertainty in this finding (HR = 0.30, 95% CI 0.10 to 1.10). Furthermore, the results reported in this article were considered to be at very high risk of bias.
In contrast, the findings in He et al.67 suggested a harmful effect of sunlight. They found that greater physician-assessed actinic skin damage was associated with a higher risk of CVD mortality (n = 8,472; CVD deaths = 1,500). Those whose skin damage was considered severe had a 64% increased risk of CVD mortality compared with those with no actinic skin damage (HR = 1.64, 95% CI 1.29 to 2.10; high risk of bias). As shown for all-cause mortality, the association does not appear to be driven by smoking, which was controlled for in the analysis.
Donneyong et al.40 looked at the self-reported frequency of outdoor recreational activity (n = 11,746; CVD deaths = 1,519). The results were mixed, with wide confidence intervals that were compatible with both a benefit and harm, and considered to be at high risk of bias.
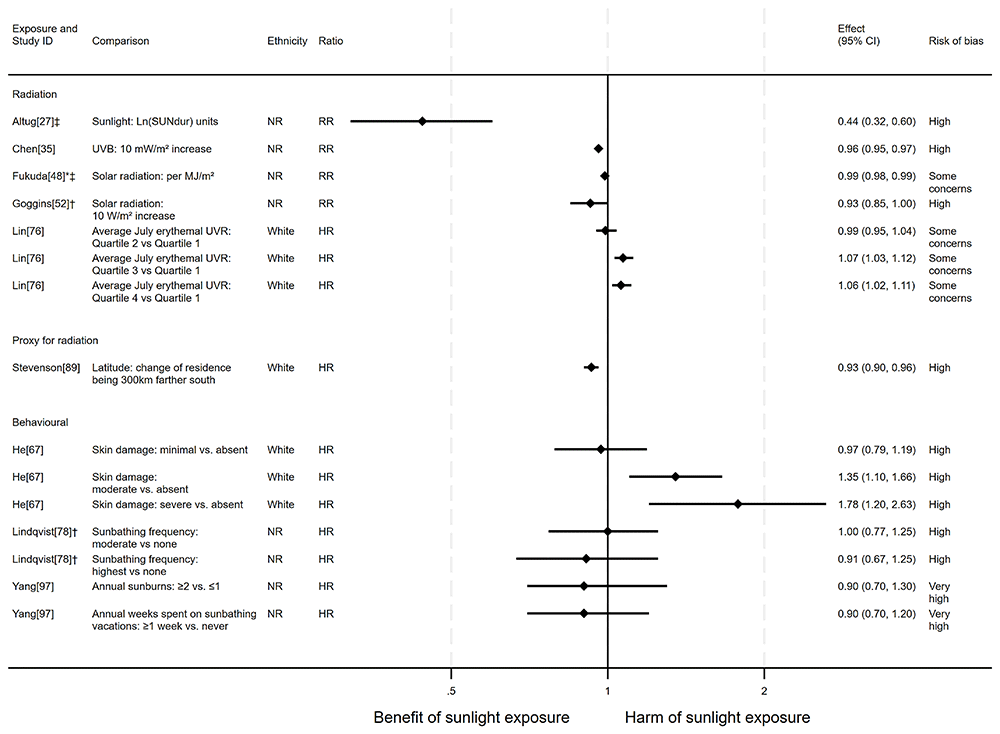
Overview. In total, 17 articles looked at the effect of sunlight exposure on all-cancer mortality, with some reporting multiple exposure types, outcomes and/or date ranges (22 analyses reported across the 17 articles). Twelve of the articles had an ecological design and five had a cohort design. The majority of analysis results (20 analyses) were in the direction of a beneficial association between sunlight and all-cancer mortality. However, two analyses67,76 suggested there may be a harmful effect of sunlight. See Figure 4 for reported relative risks and Table 1 for results reported in other formats.
*Fixed-effects meta-analysis performed to combine gender subgroups. †Result inverted in order to reflect an increase in exposure. ‡Ratio calculated via exponentiating logistic regression beta coefficient. NR: ethnicity of population not reported.
| Study | Location | Exposure | Unit of analysis | Specific Outcome | Subgroup | Analysis | Results | Direction of effect | RoB |
|---|---|---|---|---|---|---|---|---|---|
| Apperly28 | USA and Canada | Radiation (solar radiation) | Solar radiation Index | All-cancer mortality (per 100,000) | n/a | Correlation | r = -0.63 | Benefit | n/a |
| Behavioural (occupational exposure to sunlight) | % of farmers per state population | All-cancer mortality (per 100,000) | n/a | Correlation | r = -0.68 | Benefit | n/a | ||
| Camara and Brandao34 | Worldwide | Radiation (solar incidence) | kWh/m2/ day | All-cancer death rate (per 100,000) | n/a | Mortality rate | “92.48/100,000 in countries with high sunlight incidence 124.85/100,000 in countries with low sunlight incidence” (p < 0.05) | Benefit | n/a |
| Ezzati et al.42 | USA | Radiation (insolation) | Annual average solar radiation | All-cancer (smoking- related) death rate (per 10,000) | Males | Regression | β = -0.00029 (95% CI -0.00054 to -0.000031) | Benefit | Some concerns |
| Females | Regression | β = -0.00033 (95% CI -0.00051 to -0.00015) | Benefit | Some concerns | |||||
| All-cancer (non- smoking related) death rate (per 10,000) | Males | Regression | β = -0.00032 (95% CI -0.00057 to -0.000064) | Benefit | Some concerns | ||||
| Females | Regression | β = -0.00079 (95% CI -0.001 to -0.00055) | Benefit | Some concerns | |||||
| Grant and Garland56 (1950–1969) | USA | Radiation (UVB) | kJ/m2 | All- cancer ** deaths (per 100,000/year) | White males | Regression | β = -0.65 (p < 0.001) | Benefit | Some concerns |
| White females | Regression | β = -0.85 (p < 0.001) | Benefit | Some concerns | |||||
| Grant and Garland56 (1970–1994) | USA | Radiation (UVB) | kJ/m2 | All- cancer ** deaths (per 100,000/year) | White males | Regression | β = -0.54 (p < 0.001) | Benefit | Some concerns |
| White females | Regression | β = -0.82 (p < 0.001) | Benefit | Some concerns | |||||
| Grant57 | USA | Radiation (UVB) | kJ/m2 | All- cancer ** mortality rate | Black males | Regression | β = -0.34 (p = 0.01) | Benefit | Very high |
| Black females | Regression | β = -0.50 (p < 0.001) | Benefit | Very high | |||||
| Grant59 | China | Proxy for radiation (latitude) | Degree of latitude * | All-cancer deaths (per 100,000/year) | Females | Regression | β = 0.75 (p < 0.001) | Benefit | High |
| Grant62 | France | Proxy for radiation (latitude) | Degree of latitude squared * | All-cancer mortality rate | Male | Correlation | r = 0.8 (p < 0.001) | Benefit | High |
| Female | Correlation | r = 0.78 (p < 0.001) | Benefit | High | |||||
| Grant64 | USA (California) | Proxy for radiation (latitude) | Degree of latitude * | All-cancer deaths (per 100,000/year) | n/a | Regression | β = 0.47 (p = 0.009) | Benefit | High |
| Behavioural (NMSC mortality) | Mortality rate | All-cancer deaths (per 100,000/year) | n/a | Regression | β = -0.69 (p < 0.001) | Benefit | High |
Note. *An increase in latitude indicates a decrease in sunlight exposure. Therefore, a positive relationship between latitude and mortality suggests a protective effect of sunlight. **not including lung cancer. Abbreviations: β: regression beta coefficient; CI: confidence interval; n/a: not applicable; NMSC: non-melanoma skin cancer; r: correlation coefficient; RoB: risk of bias; UVB: ultraviolet B radiation.
Radiation. Ten articles looked at the effect of radiation on all-cancer mortality. The majority (n = 9) found a potentially beneficial effect of sunlight, with higher levels of radiation associated with lower levels of cancer mortality (Figure 4 and Table 1). In Altug and Kilçiksiz27, a national ecological study in Turkey, the results indicated that a one (unspecified) unit increase in sunlight duration was associated with a 56% reduction in the risk of cancer mortality (RR = 0.44, 95% CI 0.32 to 0.60; high risk of bias).
Chen et al.35 performed a national ecological study in China, looking at the effect of UVB exposure on cancer mortality. The findings suggested that a 10 mW/m2 increase in average daily UVB irradiance was associated with a 4% lower risk of mortality (rate ratio = 0.96, 95% CI 0.95 to 0.97; high risk of bias). Similarly, Fukuda et al.’s48 ecological study in Japan found that, per MJ/m2 of solar radiation, there was a 1.3% reduction in cancer mortality risk (RR = 0.99, 95% CI 0.98 to 0.99; some concerns over risk of bias). The findings in Grant and Garland56 and Grant57 suggest a similar association. Higher levels of UVB were found to be associated with lower cancer mortality rates across both Black and White males and females between 1970 and 1994 (some concerns over risk of bias). A relationship between higher levels of radiation and lower cancer mortality was also observed in Apperly28, Camara and Brandao34, Ezzati et al.42 (some concerns over risk of bias) and Grant and Garland56 (1950–1969; some concerns over risk of bias).
Goggins et al.52 found an association between higher solar radiation and lower cancer mortality, however the confidence intervals were compatible with a null effect (RR = 0.93, 95% CI 0.85 to 1; high risk of bias). In contrast, Lin et al.76 (n = 346,615; cancer deaths = 17,611) found that increasing levels of residential erythemal UVR were associated with an increased risk of cancer mortality. Comparing the fourth with the first quartile showed a 6% increase in the risk of mortality (HR = 1.06, 95% CI 1.02 to 1.11; some concerns over risk of bias).
Proxy for radiation. Four articles looked at the effect of proxy radiation exposures on cancer mortality, with all finding a potentially beneficial effect of sunlight (Figure 4 and Table 1). The findings of all four of the articles were assessed to be at high risk of bias.
Stevenson et al.89 (n = 376,719; cancer deaths = 14,125) reported that, compared with UK northerly latitudes, residences 300 km further south had a 7% reduction in mortality risk (HR = 0.93, 95% CI 0.90 to 0.96). Grant59 conducted a national ecological study in China. They found an association between higher latitudes (i.e., lower levels of sunlight) and higher cancer mortality (β = 0.75, p < 0.001). A similar relationship was found by Grant in France62 and California64.
Behavioural. Five articles studied the effect of sunlight exposure behaviour on cancer mortality (Figure 4 and Table 1). He et al.67 (n = 8,472; cancer deaths = 672) found that those with greater physician-assessed actinic skin damage had a greater risk of cancer mortality. Participants whose skin damage was considered severe had a 78% higher risk of mortality compared with those with no actinic skin damage (HR = 1.78, 95% CI 1.20 to 2.63; high risk of bias).
In contrast, Grant’s64 ecological study in California, using population-level NMSC mortality rates as a proxy for sunlight exposure, found a relationship between higher NMSC mortality rates and lower overall cancer mortality (β = −0.69, p < 0.001; high risk of bias). Apperly28 looked at the proportion of the population engaged in agricultural work and reported a relationship between higher proportions of agricultural workers and lower cancer mortality. However, no margins of error were reported with this estimate.
Two articles, Lindqvist et al.78 (n = 29,518) and Yang et al.97 (n = 38,472; cancer deaths = 457), found that recreational sunbathing behaviour was associated with lower cancer mortality. However, in both cases there were wide confidence intervals which were compatible with both a beneficial and harmful effect of sunlight exposure (Figure 4). Furthermore, they were considered to be at high and very high risk of bias, respectively.
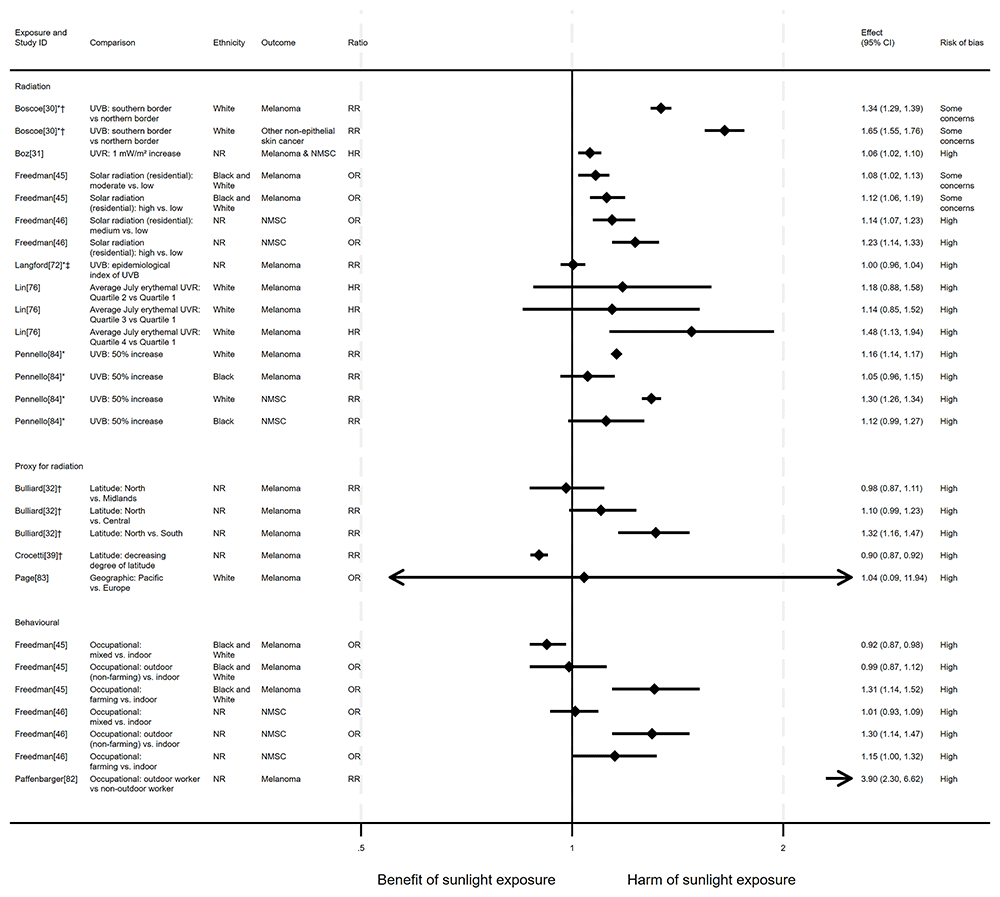
Skin cancers. In total, 23 articles investigated the effect of sunlight on skin cancer mortality with some reporting multiple exposure types, outcomes or date ranges (46 analyses reported across the 23 articles; 28 analyses measuring effect on melanoma, 16 measuring effect on NMSC, and two measuring effect on both combined). Seventeen of the articles were ecological, three had a cohort design and three used a case-control design. There were 25 analyses looking at the effect of radiation, 16 on the effect of proxy radiation exposures and five looked at the effect of sunlight exposure behaviour.
Most analysis results (n=34) suggested that higher levels of sunlight were associated with a higher risk of both melanoma and NMSC mortality (Figure 5 and Table 2). However, the results of six were in the direction of a beneficial effect of sunlight: two reported a positive relationship between degree of latitude and melanoma mortality, suggesting that those living at more northerly latitudes, where there is less sunlight, experienced higher levels of melanoma mortality25,39; two suggested an association between higher levels of UVB and lower melanoma mortality51,90; one reported a weak association between higher annual insolation and lower melanoma mortality25 and one reported an association between higher NMSC mortality rates and lower melanoma mortality58. The remaining six analyses either produced mixed results45,58,96, had no direction reported44, showed little to no effect72 or had very wide confidence intervals that were compatible with both a benefit and harm83.
*Fixed-effects meta-analysis performed to combine gender subgroups. †Result inverted in order to reflect an increase in exposure. ‡Ratio calculated via exponentiating logistic regression beta coefficient. NR: ethnicity of population not reported.
| Study | Location | Exposure | Unit of analysis | Specific Outcome | Subgroup | Analysis | Results | Direction of effect | RoB |
|---|---|---|---|---|---|---|---|---|---|
| Alcalá Ramírez del Puerto et al.25 | Spain | Radiation (irradiation) | NR | Melanoma deaths (per 100,000) | n/a | Correlation | r = 0.16 (p > 0.05) | Harm | n/a |
| NMSC deaths (per 100,000) | n/a | Correlation | r = 0.40 (p > 0.05) | Harm | n/a | ||||
| Radiation (insolation) | NR | Melanoma deaths (per 100,000) | n/a | Correlation | r = -0.113 (p > 0.05) | Benefit | n/a | ||
| NMSC deaths (per 100,000) | n/a | Correlation | r = 0.266 (p > 0.05) | Harm | n/a | ||||
| Proxy for radiation (latitude) | Degree of latitude * | Melanoma deaths (per 100,000) | n/a | Correlation | r = 0.22 (p > 0.05) | Benefit | n/a | ||
| NMSC deaths (per 100,000) | n/a | Correlation | r = -0.40 (p < 0.01) | Harm | n/a | ||||
| Elwood et al.41 | USA & Canada | Radiation (Epidemiological index of UVR) | NR | Melanoma mortality rate (per 100,000) | Males | Regression | β = 0.039 (p < 0.001) | Harm | High |
| Females | Regression | β = 0.022 (p < 0.001) | Harm | High | |||||
| NMSC mortality rate (per 100,000) | Males | Regression | β = 0.045 (p < 0.001) | Harm | High | ||||
| Females | Regression | β = 0.017 (p < 0.001) | Harm | High | |||||
| Proxy for radiation (latitude) | Degree of latitude * | All skin cancer mortality rate (per 100,000) | Males | Regression | β = -0.12 (SE = 0.011) | Harm | High | ||
| Females | Regression | β = -0.055 (SE = 0.009) | Harm | High | |||||
| Fleischer and Fleischer44 | USA | Radiation (solar radiation) | kJ/m2 | Melanoma mortality | n/a | Narrative | “No associations were demonstrated between solar energy and cancer mortality for melanoma of the skin (p = 0.60)” | NR | n/a |
| Garland et al.51 | Worldwide | Radiation (UVA) | 1 photon flux per nanometer | Melanoma mortality rate (per 100,000) | Males | Regression | β = 0.00012 (SE = 0.000050) | Harm | High |
| Females | Regression | β = 0.00004 (SE = 0.00003) | Harm | High | |||||
| Radiation (UVB) | 1 photon flux per nanometer | Melanoma mortality rate (per 100,000) | Males | Regression | β = -0.00146 (SE = 0.00094) | Benefit | High | ||
| Females | Regression | β = -0.00106 (SE = 0.00053) | Benefit | High | |||||
| Grant58 | Spain | Proxy for radiation (latitude) | Degree of latitude * | Melanoma deaths (per 100,000/year) | Males | Correlation | r = -0.08 (p > 0.05) | Mixed (gender) | n/a |
| Females | Correlation | r = 0.28 (p > 0.05) | Mixed (gender) | n/a | |||||
| NMSC deaths (per 100,000/year) | Males | Correlation | r = -0.5 (p < 0.01) | Harm | n/a | ||||
| Females | Correlation | r = -0.33 (p < 0.05) | Harm | n/a | |||||
| Behavioural (NMSC mortality) | Mortality rate | Melanoma deaths (per 100,000/year) | Males | Correlation | r = -0.03 (p > 0.05) | Benefit | n/a | ||
| Females | Correlation | r = -0.43 (p < 0.01) | Benefit | n/a | |||||
| Grant61 (1950–1969) | USA | Proxy for radiation (latitude) | Degree of latitude * | NMSC deaths (per 100,000/year) | White males | Regression | β = -0.66 (p < 0.001) | Harm | High |
| White females | Regression | β = -0.41 (p = 0.002) | Harm | High | |||||
| Grant61 (1970–1994) | USA | Proxy for radiation (latitude) | Degree of latitude * | NMSC deaths (per 100,000/year) | White males | Regression | β = -0.37 (p = 0.001) | Harm | High |
| Grant63 (1950–1969) | USA | Radiation (UVB) | kJ/m2 | Melanoma deaths (per 100,000/year) | White males | Regression | β = 0.47 (p < 0.001) | Harm | High |
| White females | Regression | β = 0.46 (p < 0.001) | Harm | High | |||||
| NMSC deaths (per 100,000/year) | White males | Regression | β = 0.34 (p < 0.001) | Harm | High | ||||
| White females | Regression | β = 0.21 (p < 0.001) | Harm | High | |||||
| Grant64 | USA (California) | Behavioural (NMSC mortality) | Mortality rate | Melanoma deaths (per 100,000/year) | White males | Regression | β = 0.46 (p = 0.03) | Harm | High |
| Lee74 (1950–1959) | USA | Proxy for radiation (latitude) | Degree of latitude * | Melanoma log mortality rate (per 100,000) | White males | Regression | β = -0.039 (95% CI -0.050 to -0.028) | Harm | High |
| White females | Regression | β = -0.037 (95% CI -0.053 to -0.021) | Harm | High | |||||
| Lee74 (1960–1969) | USA | Proxy for radiation (latitude) | Degree of latitude * | Melanoma log mortality rate (per 100,000) | White males | Regression | β = -0.037 (95% CI -0.047 to -0.028) | Harm | High |
| White females | Regression | β = -0.030 (95% CI -0.039 to -0.021) | Harm | High | |||||
| Lee74 (1970–1979) | USA | Proxy for radiation (latitude) | Degree of latitude * | Melanoma log mortality rate (per 100,000) | White males | Regression | β = -0.026 (95% CI -0.036 to -0.016) | Harm | High |
| White females | Regression | β = -0.023 (95% CI -0.032 to -0.013) | Harm | High | |||||
| Lee74 (1988–1992) | USA | Proxy for radiation (latitude) | Degree of latitude * | Melanoma log mortality rate (per 100,000) | White males | Regression | β = -0.014 (95% CI -0.023 to -0.005) | Harm | High |
| White females | Regression | β = -0.009 (95% CI -0.019 to -0.002) | Harm | High | |||||
| Rivas et al.85 | Chile | Proxy for radiation (latitude) | Degree of latitude * | Melanoma mortality rate (per 100,000) | n/a | Correlation | r = -0.88 | Harm | n/a |
| NMSC mortality rate (per 100,000) | n/a | Correlation | r = -0.53 | Harm | n/a | ||||
| Takahashi et al.90 | Japan | Radiation (UVB) | 10 J/m2/year | Melanoma mortality rate | Males | Correlation | r = -0.155 (p = 0.35) | Benefit | n/a |
| Females | Correlation | r = -0.09 (p = 0.58) | Benefit | n/a | |||||
| NMSC mortality rate | Males | Correlation | r = 0.268 (p = 0.09) | Harm | n/a | ||||
| Females | Correlation | r = 0.282 (p = 0.08) | Harm | n/a | |||||
| Wu and Weinstock96 | USA | Radiation (UV index) | UV index zone 2 vs UV index zone 1 | Keratinocyte carcinoma mortality rate | White males | Narrative | “White male KC mortality rate was found to be higher in sun Zone 2 (p = 0.004)” | Mixed (gender) | n/a |
| White females | Narrative | “There was no statistical difference between sun zones for White women (p = 0.379)” | Mixed (gender) | n/a |
Note. *An increase in latitude indicates a decrease in sunlight exposure. Therefore, a positive relationship between latitude and mortality suggests a protective effect of sunlight. Abbreviations: β: regression beta coefficient; CI: confidence interval; n/a: not applicable; KC: keratinocyte carcinoma; NMSC: non-melanoma skin cancer; NR: not reported; r: correlation coefficient; RoB: risk of bias; SE: standard error; UV: ultraviolet radiation. UVA: ultraviolet A radiation; UVB: ultraviolet B radiation.
Breast cancer mortality. In total, 11 articles looked at the effect of sunlight on breast cancer mortality, with some reporting multiple exposure types and/or date ranges (17 analyses reported, across the 11 articles). Nine articles had an ecological design and one each used cohort and case-control designs. There were ten analysis results examining the effect of radiation, four looked at proxy measures of radiation and three looked at behavioural measures of exposure. Overall, the evidence suggested that sunlight may provide a protective effect (Figure 6 and Table 3). Thirteen analysis results from Boscoe and Schymura30, Chen et al.35, Freedman et al.46, Grant and Garland56, and Grant53,57,58,62 indicated that higher levels of sunlight were associated with reduced breast cancer mortality. Three analyses from Lin et al.76, Fukuda et al.48, and Grant58 suggested that there may be a harmful association between sunlight and breast cancer mortality, and one from Fleischer and Fleischer44 was reported without a direction of effect.
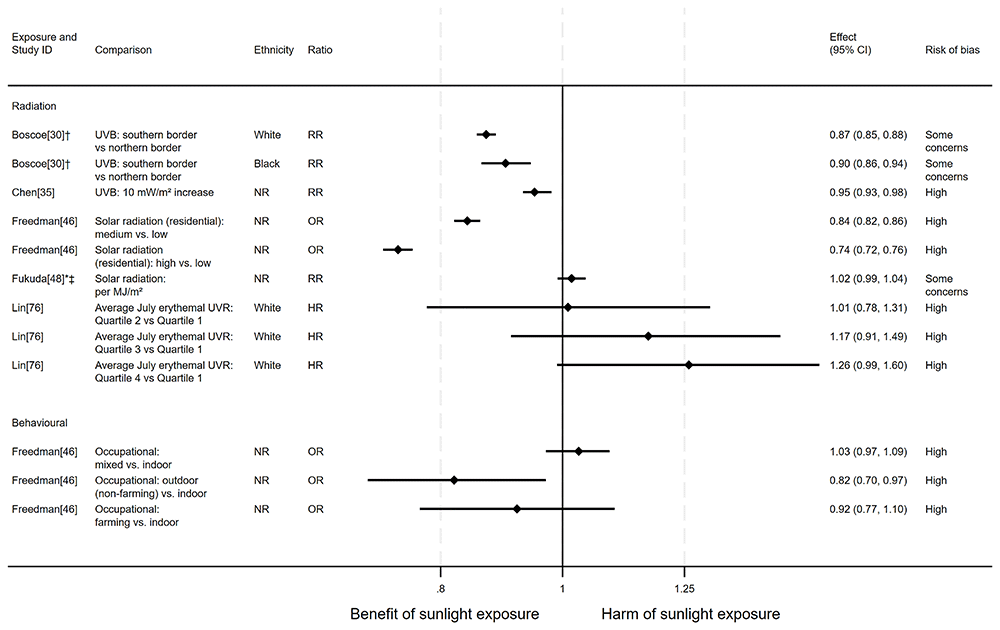
*Fixed-effects meta-analysis performed to combine gender subgroups. †Result inverted in order to reflect an increase in exposure. ‡Ratio calculated via exponentiating logistic regression beta coefficient. NR: ethnicity of population not reported.
| Study | Location | Exposure | Unit of analysis | Specific Outcome | Subgroup | Analysis | Results | Direction of effect | RoB |
|---|---|---|---|---|---|---|---|---|---|
| Fleischer and Fleischer44 | USA | Radiation(solar radiation) | kJ/m2 | Breast cancer mortality rate | n/a | Narrative | “No associations were demonstrated between solar energy and cancer mortality for breast cancer (p = 0.40)” | Not reported | n/a |
| Grant53 | Worldwide | Proxy for radiation (latitude) | Degree of latitude * | Breast cancer mortality rate (per 100,000/year) | n/a | Correlation | r = 0.66 (p < 0.001) | Benefit | n/a |
| Grant and Garland56 (1950–1969) | USA | Radiation (UVB) | kJ/m2 | Breast cancer mortality rate (per 100,000/year) | White females | Regression | β = -0.59 (p < 0.001) | Benefit | Some concerns |
| Grant and Garland56 (1970–1994) | USA | Radiation (UVB) | kJ/m2 | Breast cancer mortality rate (per 100,000/year) | White males | Regression | β = -0.71 (p = 0.006) | Benefit | Some concerns |
| White females | Regression | β = -0.71 (p < 0.001) | Benefit | Some concerns | |||||
| Grant57 | USA | Radiation (UVB DNA) | kJ/m2 | Breast cancer mortality rate | Black females | Regression | β = -0.38 (p = 0.006) | Benefit | Very high |
| Grant58 | Spain | Proxy for radiation (latitude) | Degree of latitude * | Breast cancer deaths (per 100,000/year) | Females | Correlation | r = 0.15 (p > 0.05) | Benefit | n/a |
| Behavioural (NMSC mortality and melanoma mortality) | NMSC mortality rate | Breast cancer deaths (per 100,000/year) | Females | Correlation | r = -0.38 (p < 0.01) | Benefit | n/a | ||
| Melanoma mortality rate | Breast cancer deaths (per 100,000/year) | Females | Correlation | r = 0.30 (p < 0.05) | Harm | n/a | |||
| Grant 62 (1992) | France | Proxy for radiation (latitude) | Degree of latitude * | Breast cancer mortality rate | Females | Correlation | r = 0.69 (p = 0.001) | Benefit | n/a |
| Grant 62 (1998–2000) | France | Proxy for radiation (latitude) | Degree of latitude squared * | Breast cancer mortality rate | Females | Correlation | r = 0.66 (p = 0.001) | Benefit | n/a |
Note. *An increase in latitude indicates a decrease in sunlight exposure. Therefore, a positive relationship between latitude and mortality suggests a protective effect of sunlight. Abbreviations: β: regression beta coefficient; CI: confidence interval; n/a: not applicable; NMSC: non-melanoma skin cancer; r: correlation coefficient; RoB: risk of bias; UVB: ultraviolet B radiation.
Prostate cancer mortality. There were 15 articles looking at the effect of sunlight on prostate cancer mortality, with some reporting multiple exposure types and/or date ranges (25 analyses reported across the 15 articles). There were 12 articles with an ecological design, two used a cohort and one used a case-control design. The findings were mixed (Figure 7 and Table 4). Fourteen analysis results looked at the effect of radiation, five looked at proxy radiation measures and six examined the effect of sunlight exposure behaviour. Most results suggested there may be a beneficial effect of sunlight on prostate cancer mortality (14 analyses). However, six results from Lin et al.76, Grant and Garland56, Freedman et al.46, John et al.69 and Grant58, indicated that sunlight may have a harmful effect on prostate cancer mortality. Three results from Grant58, Grant and Garland56 and Mizoue80, found little evidence of an effect; one result from Colli and Grant37 produced very wide confidence intervals compatible with both a benefit and harm and one article44 did not report the direction of effect.
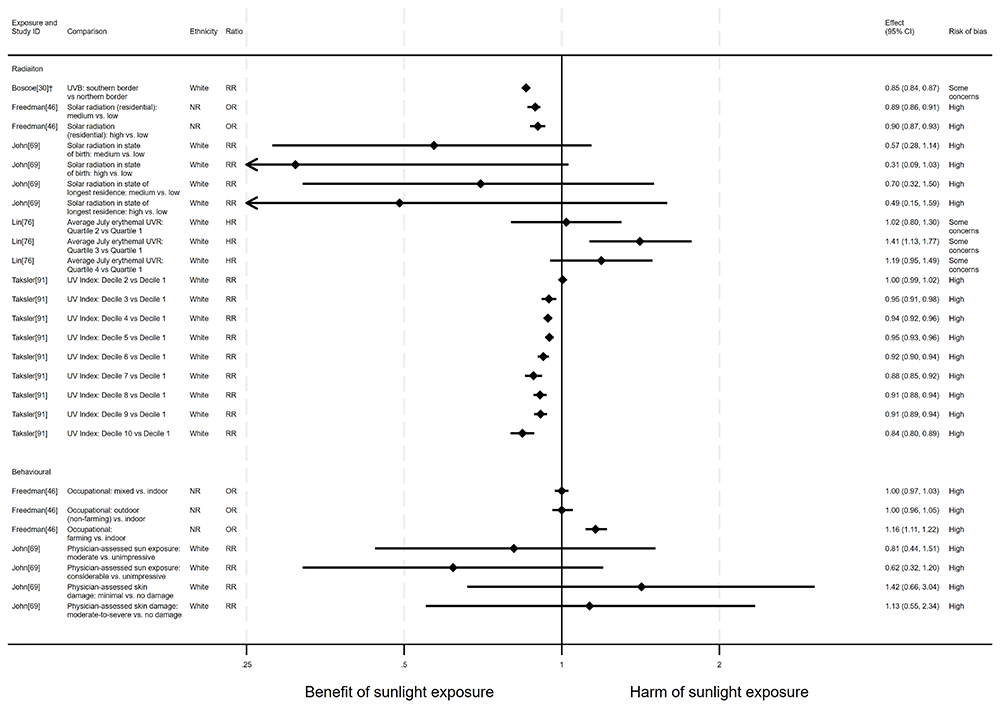
†Result inverted in order to reflect an increase in exposure. NR: ethnicity of population not reported.
| Study | Location | Exposure | Unit of analysis | Outcome | Subgroup | Analysis | Results | Direction of effect | RoB |
|---|---|---|---|---|---|---|---|---|---|
| Colli and Colli36 | Worldwide | Radiation (UV index) | UV index unit | Prostate cancer mortality (per 100,000) | Males | Regression | β = -0.81 (95% CI -1.292 to -0.322) | Benefit | High |
| Proxy for radiation (latitude) | Degree of latitude * | Prostate cancer mortality (per 100,000) | Males | Regression | β = 0.14 (95% CI 0.044 to 0.24) | Benefit | High | ||
| Colli and Grant37 | USA | Radiation (UV index) | UV index unit | Prostate cancer mortality rate | Black males | Regression | β = 0.2 (95% CI -2.4 to 2.8) | Harm | High |
| Fleischer and Fleischer44 | USA | Radiation (solar radiation) | kJ/m2 | Prostate cancer mortality rate | n/a | Narrative | “No associations were demonstrated between solar energy and cancer mortality for prostate cancer (p = 0.90)” | NR | n/a |
| Grant55 | Worldwide | Radiation (UVB) | MJ/m2/ year | Prostate cancer mortality cases (per 100,000/year) | Predominantly Caucasian populations | Narrative | Inverse relationship between UV and mortality t = -5.8, p < 0.001 | Benefit | n/a |
| Grant and Garland56 (1950–1969) | USA | Radiation (UVB) | kJ/m2 | Prostate cancer mortality rate (per 100,000/year) | White males | Regression | β = 0.02 (p = 0.94) | Harm | Some concerns |
| Proxy for radiation (latitude) | Degree of latitude * | Prostate cancer mortality rate (per 100,000/year) | White males | Regression | β = 0.52 (p = 0.09) | Benefit | High | ||
| Grant and Garland56 (1970–1994) | USA | Radiation (UVB) | kJ/m2 | Prostate cancer mortality rate (per 100,000/year) | White males | Regression | β = 0.38 (p = 0.04) | Harm | Some concerns |
| Proxy for radiation (latitude) | Degree of latitude * | Prostate cancer mortality rate (per 100,000/year) | White males | Regression | β = 0.27 (p = 0.12) | Benefit | High | ||
| Grant58 | Spain | Proxy for radiation (latitude) | Degree of latitude * | Prostate cancer deaths (per 100,000/year) | Males | Correlation | r = 0.06 (p > 0.05) | Benefit | n/a |
| Behavioural (NMSC mortality; melanoma mortality) | NMSC mortality rate | Prostate cancer deaths (per 100,000/year) | Males | Correlation | r = -0.21 (p > 0.05) | Benefit | n/a | ||
| Melanoma mortality rate | Prostate cancer deaths (per 100,000/year) | Males | Correlation | r = 0.52 (p < 0.01) | Harm | n/a | |||
| Grant62 | France | Proxy for radiation (latitude) | Degree of latitude squared * | Prostate cancer mortality rate | Males | Correlation | r = 0.68 (p = 0.001) | Benefit | n/a |
| Grant64 | USA (California) | Behavioural (NMSC mortality) | NMSC mortality rate | Prostate cancer deaths (per 100,000/year) | White males | Regression | β = -0.62 (p = 0.005) | Benefit | High |
| Mizoue80 | Japan | Radiation (solar radiation) | KWh/ Hour/day | Prostate cancer mortality (per 100,000/year) | Males | Correlation | r = -0.07 (p > 0.05) | Benefit | n/a |
| Santos Arrontes et al.86 | Spain | Radiation (sunlight) | Hours of sun exposure/year | Prostate cancer deaths (per 100,000/year) | Males | Narrative | “Mortality from prostate cancer presented statistically significant differences, being . . . lower in the areas with the greatest number of hours of sunshine per year (p = 0.041). | Benefit | n/a |
Note. *An increase in latitude indicates a decrease in sunlight exposure. Therefore, a positive relationship between latitude and mortality suggests a protective effect of sunlight. Abbreviations: β: regression beta coefficient; CI: confidence interval; n/a: not applicable; NMSC: non-melanoma skin cancer; NR: not reported r: correlation coefficient; RoB: risk of bias; UV: ultraviolet radiation; UVB: ultraviolet B radiation.
Lung cancer mortality. There were 10 articles investigating the effect of sunlight on lung cancer mortality, with some reporting multiple exposure types (12 analyses reported across the 10 articles). Nine articles had an ecological design and one had a cohort design. There were six analyses looking at the effect of radiation, three looked at proxy radiation measures and three looked at sunlight exposure behaviour. The findings were mixed (Figure 8 and Table 5). The majority of analyses (n = 7), reported in Chen et al.35, Fukuda et al.48, Fleischer and Fleischer44, Grant57,59,64, and Garland et al.50 found that higher levels of sunlight were associated with a decreased risk of lung cancer mortality. However, three analyses in Lin et al.76 and Grant58 suggested that there may be a harmful effect of sunlight. The findings of two analyses from two articles58,62 were mixed, with different findings reported for males and females.
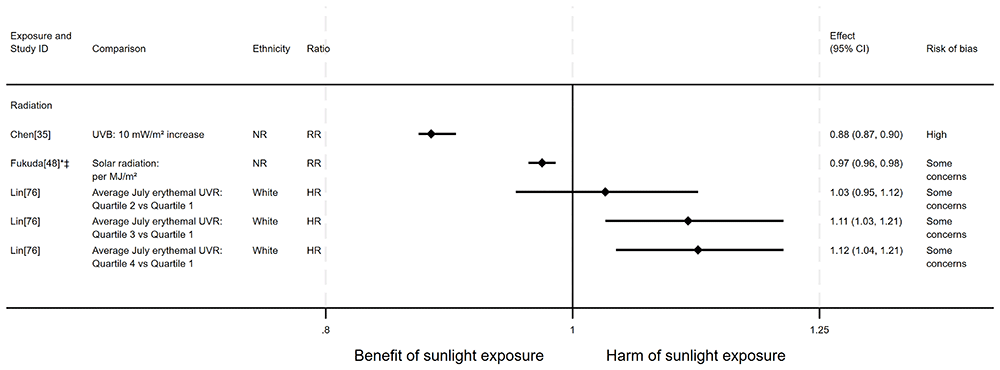
*Fixed-effects meta-analysis performed to combine gender subgroups. ‡Ratio calculated via exponentiating logistic regression beta coefficient. NR: ethnicity of population not reported.
| Study | Location | Exposure | Unit of analysis | Outcome | Subgroup | Analysis | Results | Direction of effect | RoB |
|---|---|---|---|---|---|---|---|---|---|
| Fleischer and Fleischer44 | USA | Radiation (solar radiation) | kJ/m2 | Lung cancer mortality rate | n/a | Narrative | “Associations were demonstrated between increasing solar energy and decreasing cancer incidence for lung cancer (p < 0.001)” | Benefit | n/a |
| Garland et al.50 | USA | Radiation (solar radiation) | Calories/cm² | Lung, trachea and bronchus cancer mortality rate (per 100,000) | Females | Correlation | r = -0.19 (p = 0.28) | Benefit | n/a |
| Grant57 | USA | Radiation (UVB DNA) | kJ/m2 | Lung cancer mortality rate | Black males | Regression | β = -0.52 (p = 0.003) | Benefit | Very high |
| Black females | Regression | β = -0.29 (p = 0.08) | Benefit | Very high | |||||
| Grant58 | Spain | Proxy for radiation (latitude) | Degree of latitude * | Lung cancer deaths (per 100,000/year) | Males | Correlation | r = -0.36 (p < 0.05) | Harm | n/a |
| Females | Correlation | r = -0.05 (p > 0.05) | Harm | n/a | |||||
| Behavioural (NMSC mortality; melanoma mortality) | NMSC mortality rate | Lung cancer deaths (per 100,000/year) | Males | Correlation | r = 0.02 (p > 0.05) | Mixed (gender) | n/a | ||
| Females | Correlation | r = -0.31 (p < 0.05) | Mixed (gender) | n/a | |||||
| Melanoma mortality rate | Lung cancer deaths (per 100,000/year) | Males | Correlation | r = 0.33 (p < 0.05) | Harm | n/a | |||
| Females | Correlation | r = 0.36 (p < 0.05) | Harm | n/a | |||||
| Grant59 | China | Proxy for radiation (latitude) | Degree of latitude * | Lung cancer deaths (per 100,000/year) | 35–64 year old males | Regression | β = 0.43 (p = 0.002) | Benefit | High |
| Grant62 | France | Proxy for radiation (latitude) | Degree of latitude squared * | Lung cancer mortality rate | Males | Correlation | r = 0.54 (p = 0.01) | Benefit | n/a |
| Females | Narrative | Not significant | Mixed (gender) | n/a | |||||
| Grant64 | USA (California) | Behavioural (NMSC mortality) | NMSC mortality rate | Lung cancer deaths (per 100,000/year) | White males | Regression | β = -0.38 (p = 0.11) | Mixed (gender) | High |
Note. *An increase in latitude indicates a decrease in sunlight exposure. Therefore, a positive relationship between latitude and mortality suggests a protective effect of sunlight. Abbreviations: β: regression beta coefficient; n/a: not applicable; NMSC: non-melanoma skin cancer; NR: not reported r: correlation coefficient; RoB: risk of bias; UVB DNA: DNA-weighted UVB radiation.
Bowel cancer mortality. There were 14 articles measuring the effect of sunlight on bowel cancer mortality, with some reporting multiple exposure types, outcomes and/or date ranges (31 analyses reported across the 14 articles). There were 11 articles with an ecological design, two used a cohort and one had case-control design. Sixteen analysis results examined radiation measures, eight looked at proxy radiation measures and seven looked at the effect of exposure behaviour measures. Overall, the majority of analysis results (n = 22) suggested that higher levels of sunlight were associated with a decreased risk of bowel cancer mortality (Figure 9 and Table 6). However, four analyses from Grant58 and Veach et al.94 indicated that there may be a harmful association between sunlight and mortality. Three analyses from Grant58, Lin et al.76 and Freedman et al.46 produced mixed findings, with conflicting results found either across dose levels or between gender. The result in Page et al.83 produced very wide confidence intervals that were compatible with both a benefit and a harm, whilst in Fleischer and Fleischer44 the direction of effect was not reported.
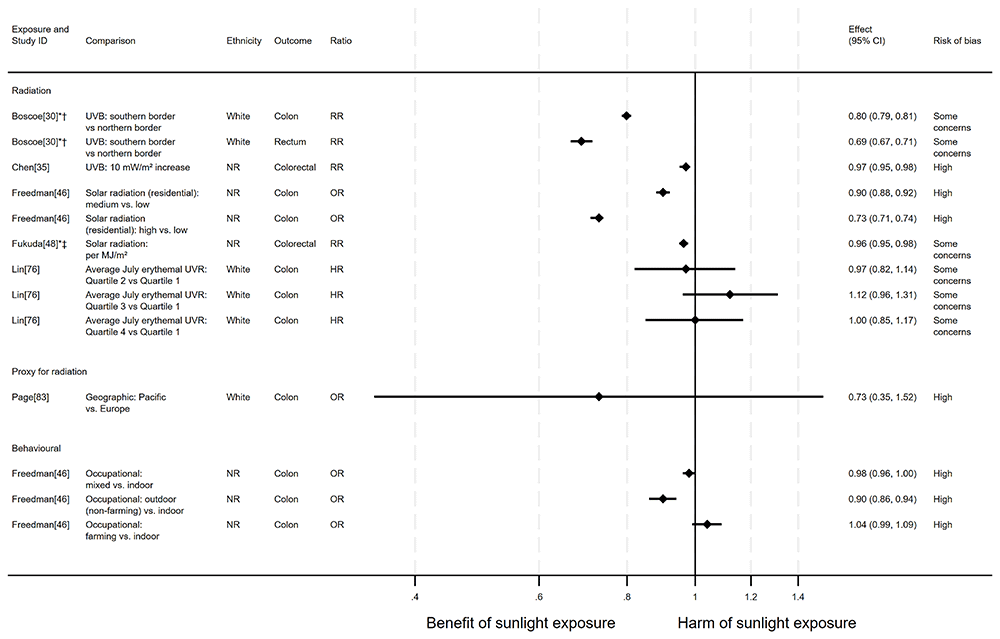
*Fixed-effects meta-analysis performed to combine gender subgroups. †Result inverted in order to reflect an increase in exposure. ‡Ratio calculated via exponentiating logistic regression beta coefficient. NR: ethnicity of population not reported.
| Study | Location | Exposure | Unit of analysis | Outcome | Subgroup | Analysis | Results | Direction of effect | RoB |
|---|---|---|---|---|---|---|---|---|---|
| Fleischer and Fleischer44 | USA | Radiation (solar radiation) | kJ/m2 | Colon/rectum cancer mortality rate | n/a | Narrative | “No associations were demonstrated between solar energy and cancer mortality for colon/ rectum cancer (p = 0.12)” | NR | n/a |
| Grant and Garland56 (1950–1969) | USA | Radiation (UVB) | kJ/m2 | Colon cancer mortality rate (per 100,000/year) | White males | Regression | β = -0.63 (p < 0.001) | Benefit | Some concerns |
| White females | Regression | β = -0.70 (p < 0.001) | Benefit | Some concerns | |||||
| Rectum cancer mortality rate (per 100,000/year) | White males | Regression | β = -0.62 (p < 0.001) | Benefit | Some concerns | ||||
| White females | Regression | β = -0.65 (p < 0.001) | Benefit | Some concerns | |||||
| Grant and Garland56 (1970–1994) | USA | Radiation (UVB) | kJ/m2 | Colon cancer mortality rate (per 100,000/year) | White males | Regression | β = -0.71 (p < 0.001) | Benefit | Some concerns |
| White females | Regression | β = -0.76 (p < 0.001) | Benefit | Some concerns | |||||
| Rectum cancer mortality rate (per 100,000/year) | White males | Regression | β = -0.75 (p < 0.001) | Benefit | Some concerns | ||||
| White females | Regression | β = -0.70 (p < 0.001) | Benefit | Some concerns | |||||
| Grant57 | USA | Radiation (UVB DNA) | kJ/m2 | Colon cancer mortality rate | Black males | Regression | β = -0.37 (p = 0.03) | Benefit | Very high |
| Black females | Regression | β = -0.05 (p = 0.76) | Benefit | Very high | |||||
| Rectum cancer mortality rate | Black males | Regression | β = -0.38 (p = 0.02) | Benefit | Very high | ||||
| Black females | Regression | β = -0.02 (p = 0.94) | Benefit | Very high | |||||
| Grant58 | Spain | Proxy for radiation (latitude) | Degree of latitude * | Colon cancer deaths (per 100,000/year) | Males | Correlation | r = 0.19 (p > 0.05) | Mixed (gender) | n/a |
| Females | Correlation | r = -0.006 (p > 0.05) | Mixed (gender) | n/a | |||||
| Rectum cancer deaths (per 100,000/year) | Males | Correlation | r = 0.61 (p < 0.01) | Benefit | n/a | ||||
| Females | Correlation | r = 0.33 (p < 0.05) | Benefit | n/a | |||||
| Behavioural (NMSC mortality; melanoma mortality) | NMSC mortality rate | Colon cancer deaths (per 100,000/year) | Males | Correlation | r = -0.30 (p < 0.05) | Benefit | n/a | ||
| Females | Correlation | r = -0.31 (p < 0.05) | Benefit | n/a | |||||
| Rectum cancer deaths (per 100,000/year) | Males | Correlation | r = -0.46 (p < 0.01) | Benefit | n/a | ||||
| Females | Correlation | r = -0.35 (p < 0.05) | Benefit | n/a | |||||
| Melanoma mortality rate | Colon cancer deaths (per 100,000/year) | Males | Correlation | r = 0.47 (p < 0.01) | Harm | n/a | |||
| Females | Correlation | r = 0.43 (p < 0.01) | Harm | n/a | |||||
| Rectum cancer deaths (per 100,000/year) | Males | Correlation | r = 0.31 (p < 0.05) | Harm | n/a | ||||
| Females | Correlation | r = 0.26 (p > 0.05) | Harm | n/a | |||||
| Grant59 | China | Proxy for radiation (latitude) | Degree of latitude * | Colorectal cancer deaths (per 100,000/year) | 35–64 years old males | Regression | β = 0.41 (p = 0.005) | Benefit | High |
| Grant62 (1992) | France | Proxy for radiation (latitude) | Degree of latitude * | Colorectal cancer mortality rate | Males | Correlation | r = 0.53 (p = 0.01) | Benefit | n/a |
| Females | Correlation | r = 0.46 (p = 0.04) | Benefit | n/a | |||||
| Grant62 (1998–2000) | France | Proxy for radiation (latitude) | Degree of latitude squared * | Colorectal cancer mortality rate | Males | Correlation | r = 0.49 (p = 0.02) | Benefit | n/a |
| Females | Correlation | r = 0.65 (p = 0.001) | Benefit | n/a | |||||
| Grant64 | USA (California) | Proxy for radiation (latitude) | Degree of latitude * | Colon cancer deaths (per 100,000/year) | White males | Regression | β = 0.47 (p = 0.01) | Benefit | High |
| Rectum cancer deaths (per 100,000/year) | White males | Regression | β = 0.48 (p = 0.007) | Benefit | High | ||||
| Behavioural (NMSC mortality) | NMSC mortality rate | Colon cancer deaths (per 100,000/year) | White males | Regression | β = -0.64 (p = 0.002) | Benefit | High | ||
| Rectum cancer deaths (per 100,000/year) | White males | Regression | β = -0.48 (p = 0.009) | Benefit | High | ||||
| Veach et al.94 | USA | Radiation (solar radiation) | Weber/m2 | Colorectal cancer mortality rate | Black males | Regression | β = 0.003 (p < 0.001) | Harm | Some concerns |
| White males | Regression | β = -0.001 (p = 0.001) | Benefit | Some concerns | |||||
| Hispanic males | Regression | β = 0.001 (p = 0.033) | Harm | Some concerns |
Note. *An increase in latitude indicates a decrease in sunlight exposure. Therefore, a positive relationship between latitude and mortality suggests a protective effect of sunlight. Abbreviations: β: regression beta coefficient; n/a: not applicable; NMSC: non-melanoma skin cancer; NR: not reported r: correlation coefficient; RoB: risk of bias; UVB: ultraviolet B radiation; UVB DNA: DNA-weighted UVB radiation.
Pancreatic cancer mortality. There were seven articles measuring the effect of sunlight on pancreatic cancer mortality, with some reporting multiple exposure types or date ranges (11 analyses reported across the seven articles). Six articles had an ecological design and one used a cohort. There were seven analyses examining the effect of radiation, two articles looked at proxy for radiation measures and two looked at sunlight exposure behaviours. The majority of analyses (n = 9) suggested that higher levels of sunlight are associated with a decreased risk of pancreatic cancer mortality, as reported in Boscoe and Schymura30, Fukuda et al.48, Lin et al.76, Neale et al.81, Grant and Garland56, and Grant58. However, one analysis in Grant58 indicated there may be a harmful association between melanoma mortality rates and pancreatic cancer mortality. One analysis in Fleischer and Fleischer44 was reported narratively, without a direction of effect (Figure 10 and Table 7).
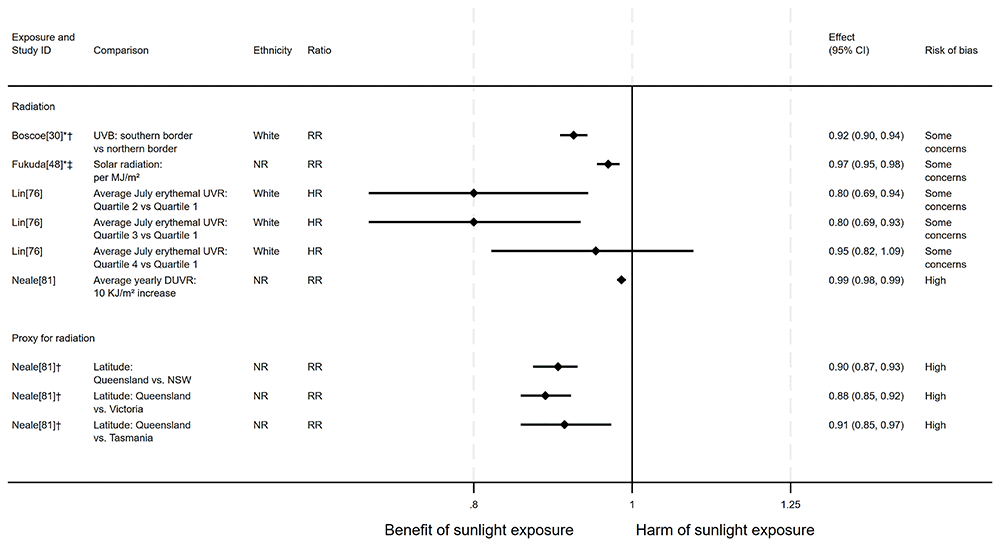
*Fixed-effects meta-analysis performed to combine gender subgroups. †Result inverted in order to reflect an increase in exposure. ‡Ratio calculated via exponentiating logistic regression beta coefficient. NR: ethnicity of population not reported.
| Study | Location | Exposure | Unit of analysis | Outcome | Subgroup | Analysis | Results | Direction of effect | RoB |
|---|---|---|---|---|---|---|---|---|---|
| Fleischer and Fleischer44 | USA | Radiation (solar radiation) | kJ/m2 | Pancreas cancer mortality rate | n/a | Narrative | “No associations were demonstrated between solar energy and cancer mortality for pancreatic cancer (p = 0.07)” | NR | n/a |
| Grant and Garland56 (1950–1969) | USA | Radiation (UVB) | kJ/m2 | Pancreas cancer mortality rate (per 100,000/year) | White males | Regression | β = -0.39 (p = 0.02) | Benefit | Some concerns |
| White females | Regression | β = -0.74 (p < 0.001) | Benefit | Some concerns | |||||
| Grant and Garland56 (1970–1994) | USA | Radiation (UVB) | kJ/m2 | Pancreas cancer mortality rate (per 100,000/year) | White males | Regression | β = -0.46 (p = 0.005) | Benefit | Some concerns |
| White females | Regression | β = -0.34 (p = 0.06) | Benefit | Some concerns | |||||
| Grant58 | Spain | Proxy for radiation (latitude) | Degree of latitude * | Pancreas cancer deaths (per 100,000/year) | Males | Correlation | r = 0.55 (p < 0.01) | Benefit | n/a |
| Females | Correlation | r = 0.40 (p < 0.01) | Benefit | n/a | |||||
| Behavioural (NMSC mortality; melanoma mortality) | NMSC mortality rate | Pancreas cancer deaths (per 100,000/year) | Males | Correlation | r = -0.35 (p < 0.05) | Benefit | n/a | ||
| Females | Correlation | r = -0.35 (p < 0.05) | Benefit | n/a | |||||
| Melanoma mortality rate | Pancreas cancer deaths (per 100,000/year) | Males | Correlation | r = 0.24 (p > 0.05) | Harm | n/a | |||
| Females | Correlation | r = 0.45 (p < 0.01) | Harm | n/a |
Note. *An increase in latitude indicates a decrease in sunlight exposure. Therefore, a positive relationship between latitude and mortality suggests a protective effect of sunlight. Abbreviations: β: regression beta coefficient; n/a: not applicable; NMSC: non-melanoma skin cancer; NR: not reported r: correlation coefficient; RoB: risk of bias; UVB: ultraviolet B radiation.
Cause-specific CVD mortality. Six articles looked at cause-specific CVD mortality (7 analyses across the 6 articles); four with an ecological design and two using a cohort design. All seven analyses examined radiation measures. Four results looked at the effect on heart disease mortality and three looked at the effect on stroke mortality. The findings were mixed (Figure 11 and Table 8). Two analyses suggested a beneficial effect of radiation on heart disease mortality42,87. However, three analyses reported a harmful effect on stroke mortality42,52,76. One produced mixed results43, and one did not report a direction of effect34 (Figure 11 and Table 8).
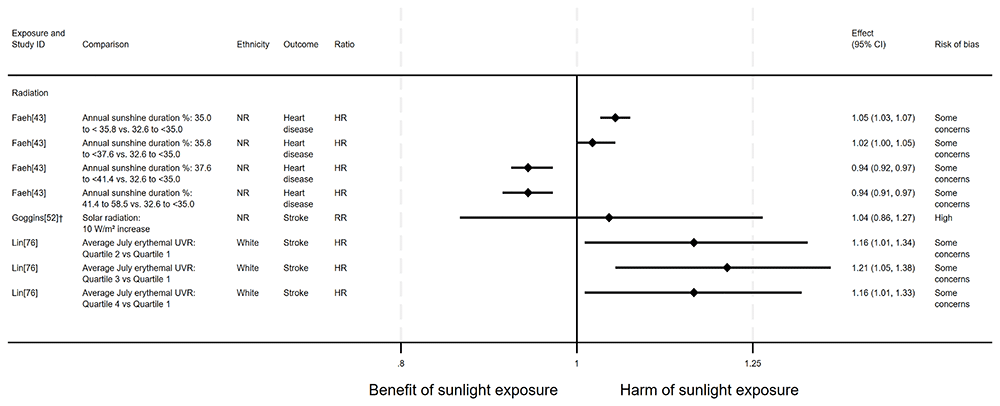
†Result inverted in order to reflect an increase in exposure. NR: ethnicity of population not reported.
| Study | Location | Exposure | Unit of analysis | Outcome | Subgroup | Analysis | Results | Direction of effect | RoB |
|---|---|---|---|---|---|---|---|---|---|
| Camara and Brandao34 | Worldwide | Radiation (Solar incidence) | kWh/m-2 / day-1 | Coronary heart disease death rate (per 100,000) | n/a | Narrative | No significant difference between high and low sunlight incidence countries (p > 0.05) | NR | n/a |
| Ezzati et al.42 | USA | Radiation (insolation) | NR | Ischaemic heart disease death rate (per 10,000) | 45+ year old males | Regression | β = -0.00023 (95% CI -0.0011 to 0.00061) | Benefit | Some concerns |
| 45+ year old females | Regression | β = -0.00014 (95% CI -0.00099 to 0.00072) | Benefit | Some concerns | |||||
| Stroke death rate (per 10,000) | 45+ year old males | Regression | β = 0.00046 (95% CI 0.0002 to 0.00072) | Harm | Some concerns | ||||
| 45+ year old females | Regression | β = 0.00062 (95% CI 0.00025 to 0.00099) | Harm | Some concerns | |||||
| Scarborough et al.87 | UK | Radiation (sunshine) | 1000s hours/ year | Coronary heart disease mortality rate (per 100,000) | Males | Regression | β = -27.3 (p < 0.05) | Benefit | Some concerns |
| Females | Regression | β = -14.3 (p < 0.05) | Benefit | Some concerns |
We sought to investigate differences in effect between people with different skin types/colours or ethnicity. However, information to allow this was limited. Most articles reported findings that examined the whole population (62%), and several limited their population to White people only (24%).
One study in the USA examined all-CVD mortality by ethnicity subgroup26, reporting slightly higher mortality risk associated with sunlight exposure for White, Black, Hispanic and Asian people, but a slightly lower risk among Native Americans. Also in the USA, Boscoe and Schymura30 found that residing along the southern border (erythemally-weighted UVB exposure of roughly 1540 kJ/m2/year) was associated with a decreased risk of breast cancer mortality compared with residing along the northern border (roughly 650 kJ/m2/year) among both White women (RR = 0.87, 95% CI 0.85 to 0.88) and Black women (RR = 0.90, 95% CI 0.86 to 0.94). Pennello et al.84 found a harmful relationship between UVB and both melanoma and NMSC mortality for both Black and White people. Finally, Veach et al.94 found that higher solar radiation (Weber/m2 by state) was associated with higher risk of bowel cancer mortality in Black (β = 0.003, p < 0.001) and Hispanic men (β = 0.001, p = 0.033), but lower risk in White men (β = −0.001, p = 0.001).
It was sometimes possible to make indirect comparisons across people of different skin types/colours or ethnicity. Grant and Garland56 and Grant57 reported data for the White population and the Black population, respectively, in the USA between 1970 and 1994. The results were suggestive of a beneficial effect of sunlight on all-cancer, breast cancer and bowel cancer mortality for both Black and White people. Boscoe and Schymura30 reported that higher levels of sunlight exposure were associated with a decreased risk of prostate cancer in White men in the USA between 1993 and 2002 (RR = 0.85, 95% CI 0.84 to 0.87); whilst conversely, Colli and Grant37 observed a small positive association between higher winter UV Index and prostate cancer mortality among Black men in the USA between 1992 and 2001, though the confidence intervals were compatible with both benefit and harm (β = 0.20; 95% CI −2.4 to 2.8).
The evidence identified by this review provides a mixed message about the association between sunlight exposure and all-cause mortality risk. Eight articles reported data for our primary outcome, with half having results in the direction of a beneficial association and half with results in the direction of a harmful association between sunlight and all-cause mortality. Mixed results were also found for all-CVD mortality, while a majority reported that higher levels of sunlight were associated with lower risk of all-cancer mortality. There was considerable uncertainty in the results across all outcomes.
As expected, most articles looking at skin cancer found that higher levels of sunlight were associated with higher levels of both melanoma and NMSC mortality. In contrast, most articles examining the five cancers with the highest UK mortality rate (breast, prostate, lung, bowel and pancreatic cancer) found that higher levels of sunlight were associated with lower risks of mortality. In particular, for pancreatic cancer, the available evidence was somewhat consistent in suggesting a beneficial effect of sunlight. Despite this, the evidence was not fully consistent for any outcome, with some findings suggesting a harmful effect of sunlight. There were also mixed findings when looking at specific causes of CVD mortality (heart disease and stroke). As with the primary outcomes, there was considerable uncertainty in the findings.
Many of the associations we observed between higher sunlight exposure and lower risk of all-cause and all-CVD mortality came from studies conducted in higher latitude countries (specifically, the UK and Sweden). On the other hand, many of the studies finding associations between higher sunlight exposure and higher risk of these mortality outcomes were conducted in the USA. These observations are consistent with the possibility that, whilst there are long established risks associated with sunlight exposure in high UVR locations, the benefits of sunlight may possibly outweigh the harms in regions with a generally low UV index10. However, this is not a conclusion we can reach with confidence from the data, given the limitations of the evidence base. Furthermore, a potentially beneficial effect of sunlight on all-cause and all-CVD mortality was observed in Hong Kong52, the location closest to the equator amongst all those included in the analyses.
It is plausible that the relationship between geographical location and effect of sunlight exposure is moderated by skin type. Those with lighter skin may experience greater benefits of sunlight in higher latitude areas with typically low UVR. For example, in this review, the beneficial effect observed on all-cause and all-CVD mortality in northern Europe77,89. Whilst, for those with darker skin, the benefits might be more pronounced in lower latitude, high UVR areas, as suggested by the beneficial effect observed for all-cancer mortality in Turkey27 and Hong Kong52. However, of these four studies, only Stevenson et al.89 was specific in reporting the skin type/colour or ethnicity of their sample (restricting inclusion to only White participants). Therefore, such theories can only be made based on broad assertions about the skin type of large populations, and so ought to be made with caution.
We intended to examine the extent to which the effects of sunlight exposure on mortality would vary according to skin type/colour or ethnicity. Evidence to allow this investigation was limited. In the four studies that reported results by these subgroups, the findings suggested some differences. For example, in the USA, the Native American population were found to have a more beneficial association between sunlight exposure and all-CVD mortality, compared with other ethnicities26. However, this result should be treated with caution as the Native American population is relatively small. Nonetheless, there were also findings suggesting similar results between those of different skin type/colour or ethnicity, such as the beneficial association between UVB and breast cancer mortality found for both Black and White women30. Given the lack of available data on skin type/colour or ethnicity, any conclusions drawn from these studies are limited.
Skin cancer mortality was the only outcome for which the evidence clearly suggested that the risks of sunlight exposure outweighed the benefits. Given that melanoma mortality is known to be lower in those with darker skin17, this highlights the need for nuanced sun exposure guidance. However, approximately a quarter of the main articles included in this review were restricted to White populations. Around two thirds reported on the whole population, though given that a large number of these were conducted in Europe and North America, it is likely that the populations in those articles were predominantly White as well. In order to gain a more complete picture of the relationship between sunlight exposure and mortality, further studies investigating the impact of skin type/colour or ethnicity are warranted. This, in turn, would help organizations responsible for sun safety messaging to provide more appropriate guidance for those with different skin types. For example, a recent summit of sun exposure experts in Australia promoted the use of sun safety advice that clearly distinguishes recommendations for people of different skin types98.
While it is well-established that sunlight increases the risk of skin cancer, particularly through UVR damage to skin cell DNA, the mechanisms through which sunlight may affect non-skin cancer risk and mortality are unclear. Sunlight exposure of the skin is usually the body’s major source of vitamin D11 and experimental studies show that vitamin D can potentially slow or prevent the development of cancer by several cellular mechanisms. These include promoting cellular differentiation and cell death, reducing cancer cell division and tumour blood vessel formation, inhibiting tumour progression and metastasis99, and stimulating the immune response to cancer cells100. Moreover, vitamin D receptors are present in many organs and cell types101. It is therefore conceivable that some of the beneficial effects of sunlight on mortality may be mediated by vitamin D. However, in randomized trials, vitamin D supplements had little to no effect on the risk of developing cancers (both overall and at specific sites) as well as on CVD and all-cause mortality102. It is possible, therefore, that the suggested beneficial effects of sunlight on cancer mortality may involve vitamin D-independent pathways.
The radiation measured by studies in this review included solar radiation (encompassing UVR, visible light (VL) and infrared radiation (IRR)) and ambient UVR (UVB and UVA), while many focused primarily on UVB, which encompasses the wavelengths initiating vitamin D synthesis in the skin, as well as being principally responsible for direct DNA damage in skin cells. However, it is now recognized that there is a range of potential benefits of solar radiation on health besides vitamin D synthesis103. The radiation responsible for these effects may include UVB and/or UVA, and possibly other types of radiation. For example, UVA and UVB are reported to regulate release of the vasodilator nitric oxide from skin cells, potentially protecting against CVD15,16. While UVB is generally more potent than UVA in effecting local skin immunomodulation, both may influence systemic immunity101. Therefore, it is warranted to consider a broader range of solar radiation and its effects with respect to mortality, than UVB alone. Apart from UVR, this includes the VL and IRR which are also emitted by the sun, and reach and penetrate the skin where they may have biological effects. For example, experimental research has indicated that VL may contribute to skin cancer development as well as having potential beneficial effects104.
Strengths of our review include our pre-specified review methodology with a comprehensive search and adoption of systematic review methods widely considered to reduce biases and human error. We included a wide range of measures of sunlight exposure, including measurements of radiation, proxy measures of radiation, and behaviour associated with sunlight exposure. Since we might expect the nature of confounding to be different for different exposures (e.g. for geographical level versus individual level exposure measures), our approach builds in the idea of ‘triangulating’ analyses that have different potential biases105. Our comprehensive approach enabled enquiry into an increasingly debated area of public health, i.e. the benefits and risks of sunlight exposure, through assessment of associations with all-cause mortality, and separately with all-cancer mortality and all-CVD mortality. We found a large number of articles and have presented the results systematically by outcome, with consideration of risk of bias, consistency of findings and imprecision.
There was considerable potential for bias in the results of the included studies. None of the results included in this review were judged to be at low risk of bias, and, in most cases, we judged the results to be at high risk of bias. The main sources of bias were a lack of control over important confounders, and the measurement of sunlight exposure. There is also a high risk of publication bias, with the potential for statistically significant associations (potentially in either direction) being considered more attractive for publication. Source of funding was poorly reported in general and we cannot exclude that conflict of interest was also associated with publication bias.
Concerns about indirectness (or applicability) of the evidence arose primarily from the measures of exposure. The measures of radiation encompassed heterogenous methodology and measurement units, ranging from satellite-derived data and ground-based measurements of radiation, to mean annual sunshine hours. Some of these measures can provide reliable estimates of ambient sunlight levels at a given location, but do not provide information about individual-level or skin exposure, which are influenced by sun exposure behaviour and sun protection measures (e.g. use of clothing, sunscreens and shade). This issue is further compounded for the proxy radiation measures, e.g., latitude. For example, studies using personal dosimeters to investigate individual-level sun exposure in northern and southern latitudes in New Zealand showed that, regardless of latitude, people received less than 2% of ambient UVR exposure106. This finding is echoed by a study in the UK showing that White people were exposed to ~2% ambient UVR, whilst those with darker skin types were exposed to even less, ~1%107. As such, the validity of latitude as a proxy measure of radiation, especially when considering skin cancer, may be limited and results should be interpreted with caution.
Furthermore, the fact that no study was conducted using a direct measurement of individual sunlight exposure makes it difficult to disentangle the specific effects of sunlight on the skin from various potential confounders. For example, people living in areas with higher levels of sunlight might experience health benefits such as increased outdoor physical activity, better diet, and improved quality of life. Moreover, the effect of such confounders are likely to vary across region and/or country, further complicating the relationship.
Some of the findings were imprecise, with wide margins of error which were often compatible with a higher and lower risk of mortality being associated with higher sunlight exposure. There was also inconsistency in the findings. For every outcome included in the review, we found results suggesting both a beneficial and a harmful effect of sunlight.
The limitations found across the current available literature lead us to several suggestions for future research. First, there is a clear need for more research into the effects of sunlight on people with darker skin. The majority of studies were conducted in predominantly White populations, with many specifically limiting inclusion to White people only. Moreover, skin type/colour or ethnicity data ought to be more commonly reported in population level studies. This would allow for more insightful analyses of the available data. For example, allowing for further investigating into the hypothesis that those with darker skin benefit most from sunlight in high UVR areas.
There is also a clear paucity of studies providing data on individual-level, personal sunlight exposure. Whilst this is difficult to achieve in practice, the combination of individual-level data on exposure, as well as important confounders, with larger population-level data would provide valuable insight into the long-term effects of sunlight exposure.
Finally, consideration should be given to standardizing sunlight measurement data. Across the studies included in the narrative synthesis, even just considering the radiation-based exposure types, there was wide variation in the methods used to measure exposure. As such, future research might focus on the development of standardized measures of exposure that can be applied consistently across various locations and populations. Alternatively, methodology could potentially be developed to convert the various measurements of radiation, as much as possible, into a common unit. This would provide a valuable extension to the current review, allowing for statistical synthesis of the mortality findings, and could subsequently be used to investigate other associated outcomes such as disease incidence.
In conclusion, evidence from existing observational epidemiological studies of the association between sunlight exposure and mortality is inconclusive. While most studies of skin cancer mortality demonstrate a higher risk associated with more exposure to sunlight, many studies of other cancers have reported lower risks associated with more exposure to sunlight. Evidence for cardiovascular mortality is mixed. Overall, the current available evidence does not indicate a large effect of sunlight on mortality. Perhaps because of a variety of effects that sunlight can have, or possibly because of potential biases in the studies available, findings for overall mortality are too variable to provide a rationale for changes to sun protection guidance.
Figshare: Dataset for “The effects of sunlight exposure on mortality: a systematic review of epidemiological studies” https://doi.org/10.6084/m9.figshare.28660109108.
The project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: Supplementary information for ““The effects of sunlight exposure on mortality: a systematic review of epidemiological studies” https://doi.org/10.6084/m9.figshare.2906965721
This project contains the following extended data:
- Appendix S1. Search strategies.
- Table S1. Characteristics of included studies.
- Table S2. Overlapping data and main article selection.
- Table S3. Description of exposures measured in articles included in analysis.
- Table S4. Risk-of-bias assessments.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: PRISMA checklist for "The effects of sunlight exposure on mortality: a systematic review of epidemiological studies" https://doi.org/10.6084/m9.figshare.28937123109.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank Monika Halicka and Christie Cabral (University of Bristol) for their contributions to writing the protocol for this review. We thank Ann R Webb (Professor of Atmospheric Radiation, University of Manchester) for her advice regarding the atmospheric radiation dosimetry. LER acknowledges the support of the NIHR Manchester Biomedical Research Centre (NIHR203308). We thank members of our public advisory panel: Sharon Bernard, Joy Bramwell, Vinette Jones and Robin Clay. Permission was obtained to include the names and affiliations of those acknowledged.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biophysics, molecular genetics, and cell biology of sunlight-induced skin cancer; photobiology.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I'm experienced in the fields of skin biology, circadian biology, photobiology, and metabolism.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Dermatology, photobiology, epidemiology, translational medicine
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology, genetic epidemiology, sun exposure and vitamin D
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Richard McKenzie is an internationally recognised expert in the measurement, understanding, and implications of solar UV radiation. He wrote the initial review, and sought further insight from Ben Liley, a fellow atmospheric scientist with particular expertise in the analysis of UV dosimeter measurements of personal exposure. James Liley also previously contributed to the latter, but is now a medically-qualified biostatistician. He has contributed to the questions on statistics, and shared insight into the public health context of the study
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
References
1. Nessvi S, Johansson L, Jopson J, Stewart A, et al.: Association of 25‐Hydroxyvitamin D3 Levels in Adult New Zealanders with Ethnicity, Skin Color and Self‐Reported Skin Sensitivity to Sun Exposure. Photochemistry and Photobiology. 2011; 87 (5): 1173-1178 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Richard McKenzie is an internationally recognised expert in the measurement, understanding, and implications of solar UV radiation. He wrote the initial review, and sought further insight from Ben Liley, a fellow atmospheric scientist with particular expertise in the analysis of UV dosimeter measurements of personal exposure. James Liley also previously contributed to the latter, but is now a medically-qualified biostatistician. He has contributed to the questions on statistics, and shared insight into the public health context of the study
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Behavioral skin cancer prevention. My only statistical concern was how the male and female data were combined in some cases.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biophysics, molecular genetics, and cell biology of sunlight-induced skin cancer; photobiology.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I'm experienced in the fields of skin biology, circadian biology, photobiology, and metabolism.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
References
1. Neale R, Beedle V, Ebeling P, Elliott T, et al.: Balancing the risks and benefits of sun exposure: A revised position statement for Australian adults. Australian and New Zealand Journal of Public Health. 2024; 48 (1). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology, genetic epidemiology, sun exposure and vitamin D
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
No
References
1. Wilde S, Timpson A, Kirsanow K, Kaiser E, et al.: Direct evidence for positive selection of skin, hair, and eye pigmentation in Europeans during the last 5,000 y. Proceedings of the National Academy of Sciences. 2014; 111 (13): 4832-4837 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Dermatology, photobiology, epidemiology, translational medicine
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
|
Version 2 (revision) 28 Nov 25 |
read | read | read | read | read | |
|
Version 1 18 Jun 25 |
read | read | read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)