Keywords
Recurrent respiratory papillomatosis (RRP) clinical registry development
Recurrent respiratory papillomatosis (RRP) is characterised by benign wart-like growths in the respiratory tract caused by the human papillomavirus (HPV). These warts vary in size and grow quickly, causing voice changes and airway obstruction. Whilst the condition is rare, RRP is more common and aggressive in children. There is currently no curative treatment for HPV, therefore RRP is managed by maintaining a safe airway and a serviceable voice by repeated surgery to remove the growths.
A lack of specific diagnostic codes prevents reliable case ascertainment of RRP from routine administrative databases such as Hospital Episode Statistics. To determine RRP burden in the UK, we conducted a cross-sectional survey of ENT consultants; 283 responded, identifying 918 RRP patients, half of whom received surgical intervention for RRP in the previous 12 months with 16 different interventions reported.
Randomised controlled trials for RRP interventions are difficult due to the rarity of the disease, variation in severity and progression and non-standard care across the NHS. Consequently, there is a lack of definitive efficacy and safety evidence. The only national guidance for RRP interventions is “Radiofrequency cold ablation for respiratory papillomatosis” (NICE IPG434, 2017) which recommended further data collection due to lack of evidence. However, due to the wide variation in RRP management across the NHS, clinical opinion favoured that any data collection should include a comparison of safety and efficacy of all RRP interventions in order to advise which improved patient outcomes and quality of life.
To address lack of evidence, and inform the future care of RRP patients, we developed a registry and used it to collect real-world data from patients receiving treatment for RRP in NHS hospitals across the UK. The purpose of this paper is to share lessons learned from this national data collection exercise to inform future clinical registry development.
Recurrent Respiratory Papillomatosis (RRP) causes wart-like growths in the airway, which make it difficult to breathe and speak. There is no known cure. Patients have their symptoms checked at hospital visits, and may need repeated surgical procedures to remove the growths and manage their symptoms. Different hospitals use a variety of different methods to manage RRP, and no one knows which methods work the best. We set up a registry to collect information from hospitals across the UK to understand more about which treatments for RRP work best for which patients. We learned a lot from the process of setting up and running this national data collection. The purpose of this paper is to share this learning to support the development of future clinical registries.
Recurrent respiratory papillomatosis (RRP) clinical registry development
Recurrent respiratory papillomatosis (RRP) is a condition characterised by benign wart-like growths in the respiratory tract, caused by the human papillomavirus (HPV)1. These warts vary in size and grow quickly, causing voice changes, chronic cough and airway obstruction2, which make it difficult to breathe, speak and lead a normal life. People with RRP are commonly misdiagnosed with asthma, croup, allergies, vocal nodules, or bronchitis, which delays diagnosis and treatment. Symptoms of RRP are managed by maintaining a safe airway and a serviceable voice by repeated surgery, usually under general anaesthesia, to remove the growths. The condition is relatively rare, but is more common and aggressive in children (1.8 per 100,000 adults, 4.3 per 100,000 children)3, and tends to recur after treatment. The results a survey of 283 ear, nose and throat (ENT) consultants in the UK, conducted by our research group recently, identified 918 RRP patients, of which 479 received at least one intervention in the previous 12 months4.
There is a lack of national guidance on how to manage the RRP condition. Controlled trials for this condition are difficult because RRP is rare, disease severity varies, it is difficult to prospectively determine the severe cases of RRP and there is no consensus exists on appropriate outcome measures. In 2012, the UK National Institute for Health and Care Excellence (NICE) issued national interventional procedure guidance which recommended the use of radiofrequency cold ablation for the treatment of RRP, under special arrangements for clinical governance, consent and audit or research5. This was due to a lack of safety and efficacy evidence (in both quantity and quality), around the use of this treatment. However, there is wide variation in the clinical management of RRP across the NHS, with the recent UK study with ENT consultants describing the use of 16 different RRP interventions. Due to general lack of evidence regarding efficacy of RRP interventions, long-term safety and RRP disease progression, the study team aimed to develop a national longitudinal data collection to inform the future care of RRP patients.
With support from the Newcastle Joint Research Office (NJRO) we secured funding from the NIHR’s Research for Patient Benefit (RfPB) programme to develop a secure online database (PB-PG-0416-20037) to capture information from 400 RRP patients. This involved a two-stage application process with a maximum funding cap of £150,000. Contributors to the grant application included the study team (clinical lead: ENT surgeon, technical lead: Head of Northern Medical Physics and Clinical Engineering (NMPCE), computer scientist, research scientist), local research nurse, local IT/Information Governance, NJRO, YPAGne, a health economist, business development manager, and Research Design Service North East. Letters of support were gained from NIHR Clinical Research Network (CRN) North East and North Cumbria, and NICE.
The grant included funding for data collection centres to support time for data entry. This reflected the desire to capture RRP care across the NHS, including smaller hospitals without dedicated ENT research nurse support. We used the model non-commercial agreement (mNCA) as a template for individual site agreements at each participating data collection centre to facilitate the transfer of funds. This caused confusion at some centres who felt the length and complexity of the agreement was disproportionate to the amount of money involved (£73 per patient entered). Setting up and managing separate agreements across 48 data collection centres was also resource intensive. To date, many centres have not invoiced regularly.
Originally, funding was secured to capture data on paediatric RRP patients only (as RRP has higher incidence in children) but clinicians advised of the importance of capturing data from adult patients who have either contracted RRP as adults or who have had the condition since childhood. A successful application to ethics and funder was made to expand the registry to include adults.
Due to higher prevalence in children, the research team engaged with the Young Persons Advisory Group – North East (YPAGne) during study design conceptualisation, prior to funding application. Feedback was incorporated into study design, the age-tailored patient information sheets, publicity to encourage recruitment and outputs of research to ensure the results were accessible to patients. YPAGne also recommended developing a study-specific patient website to update participants (and others) on study progress. Due to slow recruitment, and the expansion of the study to include adults, additional input was sought from the Newcastle Advising Patient Experience (APEX) and NIHR VOICE patient groups. An adult RRP patient and a parent of a child with RRP were recruited to the research study steering group. Early engagement with PPI groups positively influenced the study design, made the study more accessible to the target demographic and strengthened both ethics and grant applications. The study email address was added to the study website and sent to clinicians, patients and their families via posters in voice clinics across the UK (distributed on our behalf by the British Voice Association (BVA)). 18 people (patients or parents of children affected by RRP) contacted the team directly to request joining the study, which emphasised the importance of this study to people affected by RRP.
Approval from the Health Research Authority (HRA) is not required for research databases. However, to avoid the need for independent ethics review at each contributing centre, the study was submitted as a research database to the Integrated Research Application System (IRAS) to seek opinion from a Research Ethics Committee (REC). Favourable ethical opinion for the registry platform, the Airway Intervention Registry, was provided on 29th December 2014 (IRAS ref: 164160). Centres agreeing to contribute data were recruited on an ongoing basis. Although not required for this project, when submitting as a research database the team obtained delegated authority from the REC to approve others to apply and use the data. We informed REC of new centres within annual reports.
Throughout data collection, we submitted four substantial amendments to REC. These were: SA1 - broadening inclusion of all RRP interventions including adjuvant therapies and inclusion of adults; SA2 - inclusion of patient invitation letter to support recruitment and changes to patient consent form to refer to UK Data Protection Bill 2018 in line with GDPR; SA3 - extended data collection; SA4 -additional datafields to capture COVID impact. There were also four non-substantial amendments: NSA1 - permitting retrospective consent; NSA2 - extended different data collection (not RRP); NSA3 - updating reference number on study documentation; NSA4 - extended data collection.
General understanding of research databases was lacking amongst participating centres. Most R&D departments continued to review and approve the study, and requested the form of documentation typically required for controlled trials (e.g. HRA letter of approval, delegation log). A further disadvantage of research databases is the maximum length of REC opinion is five years. To extend data collection, the research team had to submit an additional IRAS application (IRAS renewal: 288078). This required the study team to change all study documentation and formally notify data collection centres. This caused a substantial and unnecessary administrative burden as the purpose and method of data collection had not changed. In hindsight, submitting to IRAS as an observational study would have required individual review at each contributing centre (which most did, in any case), but would have caused less confusion during study set up and simplified study extensions.
To link information from multiple visits over time (potentially across multiple hospitals), the registry required patient identifiers including NHS number, date of birth, gender, and partial GP postcode. Patient information sheets, consent forms and assent forms were developed for parents/guardians of children under 5 years and for different age ranges (5–11 years, 12–15 years, 16+ years). An application was made to the Newcastle upon Tyne Hospitals NHSFT Caldicott Guardian for approval to collect the data.
Following confirmation of funding, the research team prospectively registered the study (NCT03465280 on 14th March 2018). Registration with ISRCTN was delayed; NIHR confirmed that exclusively observational studies were not eligible for free registration, and when the research team paid the registration fee the registration was completed (ISRCTN36100560 on 8th May 2018).
Steps involved in developing and running a clinical registry range from defining data fields and optimising data quality, through to encouraging participation and information governance. Invaluable advice for establishing and operating patient registries is available in the Agency for Healthcare Research and Quality (AHRQ) User’s Guide6.
Involving clinicians in the registry design helped ensure that the sequence of data entry matched typical patient pathways. Data fields were defined in consultation with two ENT surgeons and one research nurse and included: demographic information, clinical history, patient and parent/guardian voice assessment questions, results from clinical examinations (including disease severity assessed by the Derkay score), histology results, details of RRP interventions (procedures and adjuvant therapies), post-procedural outcomes and long-term outcomes (including mortality). We included validation checks to improve data quality at the point of entry and minimised free text responses to avoid misinterpretation and complex free text mining during analysis. We developed draft data entry form specifications using Microsoft Word 2010 (open-source alternative: OpenOffice Writer). For each field, the specification defined its label, its format (date, number, free text, radio, multiple choice), its permitted values and whether it was mandatory. The form specifications and control flow diagrams underwent several rounds of external clinical review to ensure applicability across the NHS before handing over to the software developer.
The registry platform was created in house by NMPCE (JB) and hosted by the Newcastle upon Tyne Hospitals NHS Foundation Trust (NuTH). To protect patient data, an SSL security certificate was used to encrypt all information transmitted to and from the website. The RRP data collection was piloted for one week to test functionality and to gain feedback on ease of use from 10 ENT surgeons outside the direct research team before it officially opened on 1st April 2018. Software development was undertaken using Zend Framework 2 (an open-source PHP framework7), which enabled expansion of the Airway Intervention Registry (AIR) to incorporate the RRP data collection. AIR is hosted on the Health and Social Care Network (HSCN) and only accessible via computers connected to the HSCN. Only clinical and research staff from NHS Trusts, with access approved by their Trust’s designated principal investigator, were issued with a password-protected account to view and enter data into the online registry. This level of access is required due to the collaborative nature of ENT and respiratory departments and the urgency with which some RRP patients require treatment. It also prevented data duplication. An advantage of in-house development was that content could be modified throughout data collection, e.g. COVID data fields were introduced in July 2020 to determine impact on RRP patients.
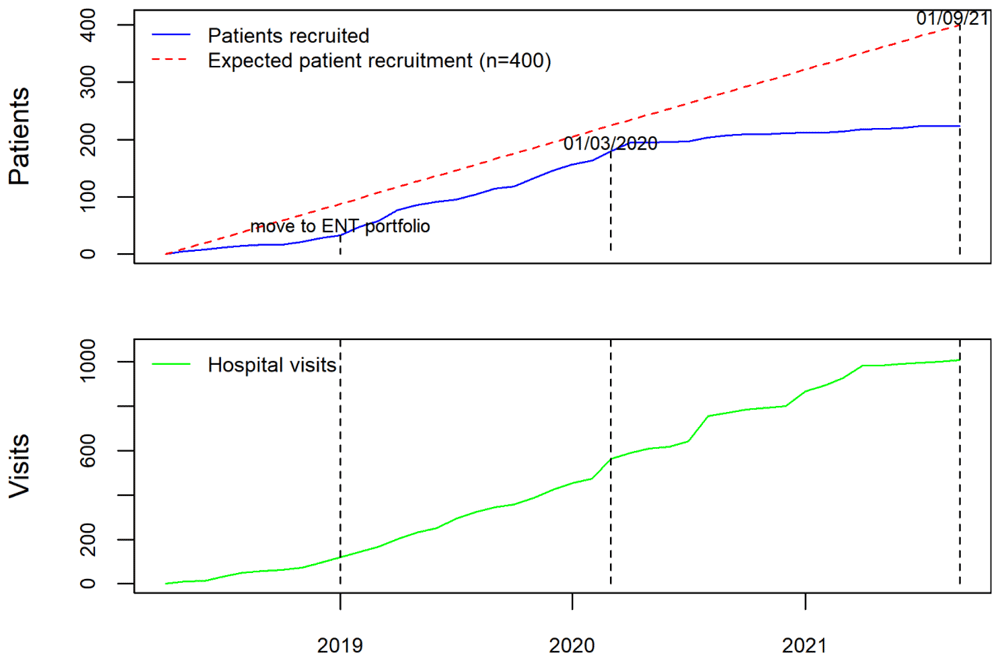
Whilst the study website (https://www.rrp.org.uk) was developed to publicise study progress, it also included a secure portal for recruited patients (or their parents/carers for younger children) to submit voice assessment questionnaires whenever they felt there had been a change in their voice quality. Patients were provided with a unique identifier (eight-character alphanumeric) during the consent process. This enabled them to contribute quality of life data to the study in a secure but convenient manner (without requiring additional hospital visits). This was of particular benefit during COVID pandemic where elective hospital attendance reduced considerably; however, this relied on recruiting centres issuing identifiers and transcribing them to the registry for data-linkage purposes (which did not always occur). To comply with the information governance requirements of patient data being submitted over the internet, an approved supplier was contracted to host the study website. The study team were provided with a secure login to update patient and procedure numbers on the website which was conducted on a weekly basis. Weekly Google analytics reports allowed the team to monitor website activity, and gauge the success of publicity efforts (Figure 1). We also worked with the website hosts to improve the site’s visibility.
The study team formed a project management group who met quarterly to discuss interim data, recruitment, safety outcomes and finances. Additionally, the registry platform had an overseeing Steering Group with membership including the study team, ENT surgeons, speech and language therapist, representation from professional societies (the British Association of Paediatric Otolaryngology, BAPO, ENTUK, and the British Laryngeal Association BLA), NICE, an adult patient with RRP and the parent of a child with RRP.
Prior to the opening of the RRP data collection, and in order to ensure that the data collected were representative of practice across UK, the study team invited all acute NHS trusts with ENT departments to contribute as data collection centres. The study was publicised by three ENT professional societies (ENTUK, BLA, BAPO) who also signed a joint statement of support for the registry and encouraged their members to participate in the study. The study team liaised directly with clinicians and R&D departments at Centres to address issues with study set up and data entry. Regular newsletters were sent to all registered users, including personalised letters, which acknowledged their contribution to the registry for appraisal and re-validation purposes. The study team worked with local CRN (NENC) to publicise the study across the national network. When the study was expanded to include adults, the main speciality on the central portfolio management system (CPMS) changed from children to ENT, which resulted in a significant boost to recruitment (Figure 2). These efforts led to 48 NHS Trusts or Health Boards joining as formal data collection centres (39 in England, four in Scotland, four in Wales, and one in Northern Ireland).
Additional publicity activities promoted the study website directly to patients. The BVA distributed leaflets at voice clinics and circulated a link to the study website to their membership (a mix of professionals and members of the public, all with an interest in promoting ‘voice’). The RRP data collection was also presented at the annual ‘Choice for Voice’ conference in September 2021. Further organisations agreed to post a link to the study website on their websites or Facebook pages: The Royal College of Speech and Language Therapists (RCSLT) published information in their Bulletin section, RRP patient support group, the RRP Foundation, shared information with their membership via their website and Facebook pages.. The study was registered on Orphanet, a discussion thread was posted on Mumsnet and the study website was promoted in the NuTH research twitter feed.
The study website was presented at the NIHR portfolio development day. Participants were encouraged to discuss the website with their patients and to give them the unique ID needed to submit voice quality questionnaires via the portal. The website was commended in the BMA 2019 Patient Information awards.
As with many research studies, the pandemic has had a significant impact on patient recruitment due to the focus of research efforts and staff on COVID-related research. Due to this, NIHR approved an extension to data collection until 31st August 2022. Of the 48 data collection centres, five have closed recruitment due to lack of capacity. However, data entry has remained open with centres contributing multiple hospital visits enabling longitudinal patient records capturing RRP outcomes over time. As of 16th November 2021, we have collected information from 256 patients including 1057 hospital attendances, which represents the largest UK RRP data collection. Data has been contributed by ENT surgeons and respiratory physicians, registrars, fellows, research nurses, speech and language therapists, data managers, patients, and their families, making AIR RRP a comprehensive and collaborative ENT registry.
There are no underlying data associated with this article.
The Airway Intervention Registry gained favourable ethical opinion as a research database on 29th December 2014 (IRAS for original application: 164160, renewal: 288078). Patients (or parents for participants under 16 years of age) are required to give written informed consent before their details can be entered into the online database. Age-appropriate Patient Information Sheets have been developed to assist with this.
AJS: Conceptualisation, Formal analysis, Funding acquisition, Methodology, Resources, Software, Supervision, Visualisation, Writing Original Draft Preparation, Writing – Review & Editing
KK: Conceptualisation, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Validation, Visualisation, Writing Original Draft Preparation, Writing – Review & Editing
EB: Data curation, Funding acquisition, Methodology, Project administration, Resources, Visualisation, Writing Original Draft Preparation, Writing – Review & Editing
JB: Conceptualisation, Data curation, Formal analysis, Funding acquisition, Methodology, Resources, Software, Visualisation, Writing Original Draft Preparation, Writing – Review & Editing
LB: Funding acquisition, Methodology, Writing Original Draft Preparation, Writing – Review & Editing
AJ: Conceptualisation, Methodology, Writing Original Draft Preparation, Writing – Review & Editing
SP: Conceptualisation, Investigation, Methodology, Writing Original Draft Preparation, Writing – Review & Editing
SJ: Investigation, Methodology, Writing Original Draft Preparation, Writing – Review & Editing
AD: Conceptualisation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Visualisation, Writing Original Draft Preparation, Writing – Review & Editing
We are grateful to The Young Persons Advisory Group North of England (YPAGne) for their contribution to the study design, recruitment approach, and publicity of results and to Newcastle Advising Patient Experience (APEX) and NIHR VOICE patient groups for their contribution to recruitment approach and publicity of results.
We are grateful to the British Voice Association (BVA), the RRPF (Recurrent Respiratory Papillomatosis Foundation), the Royal College of Speech and Language Therapists, ENT UK, the British Laryngological Association (BLA), the British Association for Paediatric Otolaryngology (BAPO) and Orphanet for their support to study publicity.
We are grateful for the support of staff at centres contributing to the Airway Intervention Registry Recurrent Respiratory Papillomatosis (AIR:RRP) data collection: Alder Hey Children’s NHS Foundation Trust; Barts Health NHS Trust; Betsi Cadawaladr University Health; Birmingham Children’s Hospital NHS Foundation; Blackpool Teaching Hospitals NHS Foundation Trust; Bradford Teaching Hospitals NHS Foundation Trust; Cambridge University Hospitals NHS Foundation Trust; Cardiff and Vale University HB; County Durham and Darlington NHS Foundation Trust; East Sussex Healthcare NHS Trust; Frimley Park Hospital NHS Foundation Trust; Great Ormond Street Hospital for Children NHS FT; Imperial College Healthcare NHS Trust; Isle of Wight NHS Trust; Lewisham and Greenwich NHS Trust; Liverpool University Hospitals NHS Foundation Trust; London North West Healthcare NHS Trust; Manchester University NHS Foundation Trust; NHS Grampian; NHS Lothian; NHS Tayside; North Cumbria Integrated Care NHS Foundation Trust; Nottingham University Hospitals NHS Trust; Pennine Acute Hospitals NHS Trust; Salford Royal NHS FT; Sheffield Children’s NHS Foundation Trust; Shrewsbury and Telford Hospital NHS Trust; South Tyneside and Sunderland NHS Foundation Trust; St George’s Healthcare NHS Trust; Stockport NHS Foundation Trust; Swansea Bay University Health Board; The Newcastle Upon Tyne Hospitals NHS FT; University College London Hospitals NHS Foundation Trust; University Hospitals Birmingham NHS Foundation Trust; University Hospitals Plymouth NHS Trust; Wirral University Teaching Hospital NHS FT.
Thanks to Edward O’Toole, the Newcastle Upon Tyne Hospitals NHS FT for his work analysing the Google Analytics reports.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical research in voice, airway, and swallowing including RRP
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Voice disorders, Recurrent Respiratory Papillomatosis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 12 Jan 23 |
read | |
|
Version 1 24 Feb 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Reviewer 2
In this manuscript the authors outline their experience setting up a national database for collection of RRP ... Continue reading Response to reviewer 2 (addressed in version uploaded on 21/12/2022).
Reviewer 2
In this manuscript the authors outline their experience setting up a national database for collection of RRP data across the UK. There is no data to be analysed nor results.
Many of the specific regulatory barriers the authors encountered appear to be specific the NHS and may not be generalizable to all countries. For example, I am rather surprised that ethical approval is not required for databases wherein patient specific identifiers are collected.
Is there any plan to continue to monitor RRP cases prospectively? To see changes in incidence with the HPV vaccine etc?
Response:
Results from the registry have been written up in a separate manuscript and will be submitted to an ENT journal.
A hyperlink has been added the the “Ethics and information governance” subsection, to support that establishment of research databases do not require HRA approval within the NHS.
An additional sentence has been added to the “Registry specification and design” subsection to highlight that the registry collected longitudinal data from recruited RRP patients (adult and children) including data from outpatient appointments, A&E attendance, voice assessments, endoscopic examination results, details of subsequent surgical interventions and adjuvant therapies.
Public and patient involvement
- I applaud the authors for involving a patient and a parent. Was there any thought in using social media?
Response: Additional text moved into PPI section (was previously present in the ongoing recruitment section). This includes discussion of how the publicity incorporated social media.Ethics and governance
- The administrative hurdles appear to be very specific to the NHS. Were there other disease specific databases from other specialties upon which the RRP database could have been modelled?
Response: The hurdles were specific to the UK (rather than to the NHS) because that was its setting. We based the register design on previous experience with surgical registries linked to routine data. We have added material to the first paragraph of the registry specification and design section to explain this point.Registry specification
- Please elaborate on "post-procedural outcomes and long-term outcomes." Does this mean surgical complications? Voice outcomes? Inter-surgical intervals?
Response: Additional outcomes added.Research study website
- Direct patient upload of voice questionnaires has been very useful in other studies including, the initially North American Airway Collaborative.
Response: Thank you for your comment. No change required.Reviewer 2
In this manuscript the authors outline their experience setting up a national database for collection of RRP data across the UK. There is no data to be analysed nor results.
Many of the specific regulatory barriers the authors encountered appear to be specific the NHS and may not be generalizable to all countries. For example, I am rather surprised that ethical approval is not required for databases wherein patient specific identifiers are collected.
Is there any plan to continue to monitor RRP cases prospectively? To see changes in incidence with the HPV vaccine etc?
Response:
Results from the registry have been written up in a separate manuscript and will be submitted to an ENT journal.
A hyperlink has been added the the “Ethics and information governance” subsection, to support that establishment of research databases do not require HRA approval within the NHS.
An additional sentence has been added to the “Registry specification and design” subsection to highlight that the registry collected longitudinal data from recruited RRP patients (adult and children) including data from outpatient appointments, A&E attendance, voice assessments, endoscopic examination results, details of subsequent surgical interventions and adjuvant therapies.
Public and patient involvement
- I applaud the authors for involving a patient and a parent. Was there any thought in using social media?
Response: Additional text moved into PPI section (was previously present in the ongoing recruitment section). This includes discussion of how the publicity incorporated social media.Ethics and governance
- The administrative hurdles appear to be very specific to the NHS. Were there other disease specific databases from other specialties upon which the RRP database could have been modelled?
Response: The hurdles were specific to the UK (rather than to the NHS) because that was its setting. We based the register design on previous experience with surgical registries linked to routine data. We have added material to the first paragraph of the registry specification and design section to explain this point.Registry specification
- Please elaborate on "post-procedural outcomes and long-term outcomes." Does this mean surgical complications? Voice outcomes? Inter-surgical intervals?
Response: Additional outcomes added.Research study website
- Direct patient upload of voice questionnaires has been very useful in other studies including, the initially North American Airway Collaborative.
Response: Thank you for your comment. No change required.