Keywords
Vaccines, Vaccine effectiveness, Vaccine impact, Vulnerable communities, Community engagement, Health equity, Uganda, Kenya
Vaccination is an important public health intervention, but not everyone benefits equally. Biological, social and structural factors render some communities vulnerable and unable to secure optimal health benefits from vaccination programmes. This drives health inequity and undermines wider vaccine impact by allowing the persistence of non-immune communities as foci for recurrent disease outbreaks. The NIHR Global Health Research Group on Vaccines for vulnerable people in Africa (VAnguard) aims to understand how biological, social, and structural factors interact to impair vaccine impact in vulnerable African communities.
The VAnguard project will be implemented through three thematic work packages (1-3) and four cross-cutting work packages (4-7). Work package 1 will investigate the biological drivers and mechanisms of population differences in vaccine responses. Work package 2 will support the understanding of how structural, social and biological determinants of vaccine response interrelate to determine vaccine impact. Work package 3 will synthesise data and lead analyses to develop, model and test community-based integrated strategies to optimise vaccine access, uptake and effectiveness. Work package 4 will plan and implement field investigations (community survey and qualitative studies (with support of work package 2) to explore structural, social & biological determinants impairing vaccine impact. Work package 5 will collaborate with work packages 1-4, to engage communities in designing interventions that aim to directly optimise vaccine impact through a process of co-learning and co-creation between them and the researchers. Work package 6 will build capacity for, and a culture of, consultative, collaborative multidisciplinary vaccine research in East Africa. Work package 7 will support the overall project management and governance. Following the project inception on the 1st of September 2022, project launch was held in November 2022.
Results from this project will contribute to the development of integrated strategies that will optimise vaccine benefits and drive health equity.
Vaccination is an important public health intervention but not everyone benefits equally. Some vaccines give weaker protection in people from rural, tropical settings than in those from high income settings. Some new vaccines, under development, also elicit weaker responses in people living in low-income, rural settings. The biological reasons for this are not fully understood. Also, some people benefit less from vaccines for socioeconomic reasons, such as the social context of the communities they live in, including limited access to accurate information to aid vaccine choices. Social and biological factors can interact to make communities “vulnerable” in terms of vaccine impact. This needs to be addressed to promote health equity, but also to secure maximum global benefit from vaccines.
VAnguard’s goal is to understand how biological, social, and structural factors interact to impair vaccine impact in vulnerable African communities, and to develop integrated strategies to optimise vaccine benefits and drive health equity.
First, we shall work with national stakeholders (such as Ministries of Health, and vaccine-related non-governmental organisations), review literature, and work on samples from previous studies, to identify Ugandan and Kenyan communities likely to have the most difficulty in getting the best out of vaccination programmes (“vulnerable communities”). Then, with stakeholders and communities, we shall co-design the VAnguard Community Study, and implement it to investigate in detail which biological and social factors most influence vaccine impact in vulnerable communities. Data and economic modellers will study the results to identify which factors could usefully be modified, and we shall work with the communities to explore ways in which this could be done. Hence, we shall co-develop strategies which national stakeholders may be able to implement straight away, or which can be tested in future studies.
Vaccines, Vaccine effectiveness, Vaccine impact, Vulnerable communities, Community engagement, Health equity, Uganda, Kenya
 How to cite: Zirimenya L, Zalwango F, Owino EA et al. NIHR Global Health Research Group on Vaccines for vulnerable people in Africa (VAnguard): Concept and Launch event report [version 1; peer review: 2 approved with reservations]. NIHR Open Res 2023, 3:35 (https://doi.org/10.3310/nihropenres.13417.1)
First published: 27 Jun 2023, 3:35 (https://doi.org/10.3310/nihropenres.13417.1)
Latest published: 26 Feb 2024, 3:35 (https://doi.org/10.3310/nihropenres.13417.2)
How to cite: Zirimenya L, Zalwango F, Owino EA et al. NIHR Global Health Research Group on Vaccines for vulnerable people in Africa (VAnguard): Concept and Launch event report [version 1; peer review: 2 approved with reservations]. NIHR Open Res 2023, 3:35 (https://doi.org/10.3310/nihropenres.13417.1)
First published: 27 Jun 2023, 3:35 (https://doi.org/10.3310/nihropenres.13417.1)
Latest published: 26 Feb 2024, 3:35 (https://doi.org/10.3310/nihropenres.13417.2)
Vaccines are among the most successful public health interventions1. This fact has achieved huge prominence and recognition during recent Ebola outbreaks and the SARS-CoV-2 pandemic. However, not all communities benefit equally. Biological and social factors interact together and with structural factors (environmental, geographical, and political circumstances outside an individual’s control) interact to reduce vaccine effectiveness and impact in vulnerable communities (Figure 1). Impaired vaccine immunogenicity is characteristic of low-income, rural populations, but the underlying mechanisms for this are not fully known2–8.
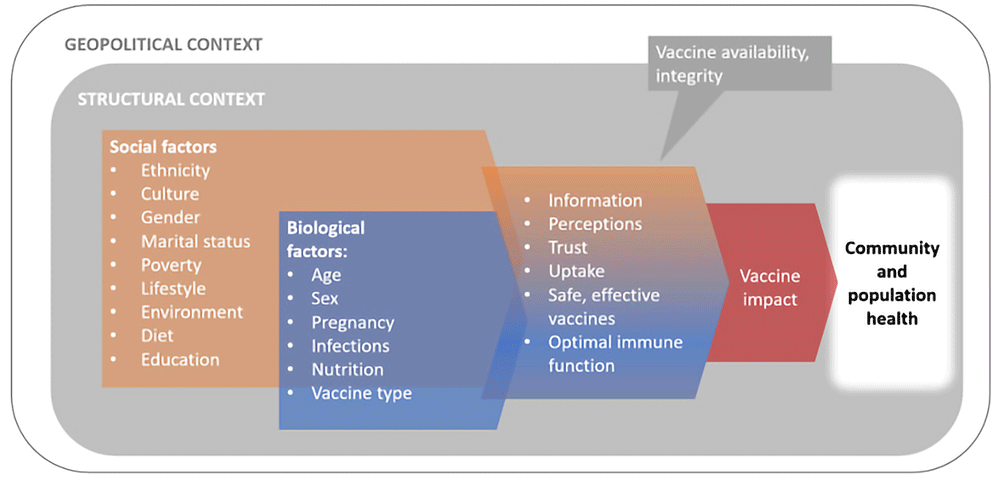
Multiple vulnerabilities can be experienced producing local, national, and global health inequities9–15.
Research suggests that a combination of “structural violence” and vulnerabilities impact vaccine uptake and access. Structural violence is suffering caused by the structure and institutions of a society that puts people in harm’s way or prevents them from meeting their needs16,17. Structurally vulnerable populations experience limited options and choices as these are constrained by the context they inhabit11,13,15. During the COVID-19 pandemic, structural vulnerabilities, particularly related to race and ethnicity, have become more apparent18.
Health is intertwined with situations in which people are born, grow up, work and age: situations that are shaped by structural inequities in power and resources. Discriminatory or inequitable policies and practices that do not take into account the impact of the prevailing context on socially constructed categories such as gender and ethnicity, influencing the ability of individuals and communities to access adequate nutrition, water and sanitation, shaping exposure to pathogenic and non-pathogenic organisms. Socially, the response to vaccination campaigns reveals hesitancy, increasingly influenced by rumours, mis- or dis-information and distrust of authorities, mediated and amplified by social and conventional media, compounding impediments to vaccine access and uptake already prevalent in vulnerable settings19. Nutritional status and infection exposure interact with an individual’s biological characteristics (e.g., genetics, age) influencing their immunological and overall health status. The experiences of humans cannot be understood by prioritising a single characteristic over another or conceptualised as additive binary factors14,20.
The SARS-CoV-2 pandemic has triggered unprecedented political support for vaccine development. People in Africa are reacting against dependence on developers and manufacturers in other continents who may prioritise their own national and commercial needs over those of more distant, poorer countries. African governments (Uganda, Kenya, Nigeria, Algeria, Egypt, South Africa) has invested in SARS-CoV-2 vaccine development; Africa’s Association of Research Universities is leading a vaccine initiative. However, whether produced in Africa or elsewhere, vaccines will not achieve their full potential for health benefit unless structural factors that impair vaccine impact among vulnerable communities are addressed. Therefore, the VAnguard group aims to understand how biological, social, and structural factors interact to impair vaccine impact in vulnerable African communities.
As a first step towards the project implementation, we held a project launch meeting in Entebbe, Uganda and in Kilifi, Kenya, in November 2022, focused on bringing together major stakeholders in the two countries. Meeting participants included representatives of the Ministry of Health (MoH) Expanded Programmes on Immunisation (EPI) in Uganda and Kenya; researchers and community representatives from both Uganda and Kenya and the VAnguard project team from the partner institutions namely London School of Hygiene and Tropical Medicine (LSHTM) (Lead), UVRI (Joint Lead), KEMRI Wellcome Trust Research Programme, MRC/UVRI and LSHTM Uganda Research Unit, Makerere University, Uganda Christian University, MoH, Uganda and Kenya, and the University of Oxford and University of Cambridge. We hereby present the VAnguard Group concept and proceedings of the launch event.
Engagement is integral to the VAnguard research cycle. Detailed stakeholder mapping will be undertaken (Work Package 5). Key national stakeholders include Ministries; non-governmental, faith-based, cultural, international bodies; Media. Initial Ministry of Health engagement instigated the proposed collaboration on vaccine-related communication.
A project specific communication and engagement strategy has been developed to guide participant and public involvement. This strategy lays out how the project plans to communicate and engage key stakeholders and communities on vaccines, throughout the project. The strategy draws on existing institutional frameworks. It includes a communication plan for the project’s internal communication and a communication and engagement plan for the project’s external audiences which include vulnerable communities in Kenya and Uganda. In each project site, identified community members will be brought on board at the earliest opportunity, to be an integral part in the project. Community representatives will confirm identified communities as a true reflection of vulnerability and add any that may have been missed by national stakeholders (WP4 and 2). The process will lead to a shared decision regarding communities to be included in the VAnguard Community Study (WP4). Community views about the project will be sought through meaningful engagement, using a range of participatory and deliberative approaches including mixed research methods (supported by WP2) to engage vulnerable populations, community representatives (Community Advisory Board members), local leaders, local department of health stakeholders, among other key groups that will be iteratively identified. Meetings will be organized at the beginning of the project and will involve District/County Health Managers, community representatives at the local site level, religious leaders and other civil society actors and influential personalities to be identified iteratively in the communities. Views from these meetings will inform the VAnguard Community Study design, including collaboration with WP2 and WP4 in the development of “knowledge, attitudes and practice” (KAP) elements of the study questionnaire (WP4).
The overall goal of VAnguard is to identify modifiable structural, social, and biological determinants of impaired vaccine impact in vulnerable African communities to inform the development of integrated strategies to drive health equity.
The specific objectives are, in Uganda and Kenya, to
1. Investigate biological drivers and mechanisms of population differences in vaccine response.
2. Understand how structural, social, and biological determinants of vaccine response interrelate to determine vaccine impact.
3. Identify and model integrated strategies to inform the development of future interventions to optimise vaccine impact among vulnerable populations.
Integrated within the project, are two supporting objectives, to
4. Empower vulnerable communities to optimise vaccine impact for their people through a process of co-learning and co-creation between them and researchers, and
5. Build capacity for, and a culture of, consultative, collaborative multidisciplinary vaccine research in East Africa
To address these objectives, implementation will be done through three thematic work packages (WPs 1, 2 and 3) and four cross-cutting WPs (4, 5, 6 and 7) (Figure 2).
Broadly, the work will comprise a preparatory phase (year 1), a VAnguard Community Study (WP4, year 2) and an analytic and intervention co-development phase (years 3 and 4). Stakeholder and community engagement will be fully integrated throughout the research cycle. During the preparatory phase, concurrent activities will be undertaken by multiple WPs. Work will be done with stakeholders (Ministries of Health, immunisation programme partners, communities) to purposely select Ugandan and Kenyan communities that are vulnerable with regard to vaccine impact (based on vaccine coverage, and on hypothesised structural, social and biological barriers to effective immunisation) and less vulnerable communities for comparison. At the same time, we shall review the literature; and undertake analyses of data and samples from previous studies to identify biological mechanisms of impaired vaccine response. The resulting information will be shared at a consultative meeting to co-design the protocol for a multidisciplinary VAnguard Community Study with up to eight communities in Uganda and Kenya (year 2, WP4). In each community, local stakeholders and community members will be worked with closely. Results from WP1, 2 and 4 will be analysed and synthesised into a modelling framework (WP3) to co-design intervention packages for testing in further trials.
The purpose of WP1 is to address Objective 1, “to investigate the biological drivers and mechanisms of population differences in vaccine responses” through the following activities.
Data and literature review. The goal of this activity will be to identify modifiable extrinsic factors specifically relevant to vulnerable communities in Africa. The review will build on earlier published work including 2019 Zimmerman & Curtis review of factors that affect vaccine immunogenicity21; a meta-analysis on the effects of “parasites” (broadly defined; both humans and other mammals) by Wait et al. in 202022; a review on nutritional factors by Savy et al. in 200923; and a more recent review by Natukunda et al. in 2022 on the effect of helminths on vaccine responses24. Many African populations are heavily exposed to parasites and other infections, such as cytomegalovirus (CMV), from a young age; some relevant nutritional deficiencies (e.g. iron) are more common in Africa than elsewhere25,26. Evidence on the quantitative effects of these factors and related interventions will be reviewed. To inform the VAnguard Community Study design (WP4), WP1 will aim to identify factors that have an important impact and offer benefits from intervention.
National stakeholders will be engaged to identify communities with high prevalence, and hence greatest potential vulnerability, regarding these parameters.
Different types of vaccine (live, viral-vectored, inert, oral, parenteral) differ in susceptibility to extrinsic exposures. Our current work on the effects of helminths indicates adverse effects for live vaccines (BCG, measles) and Hepatitis B vaccine, but not for other vaccines24. WP1 will contribute towards identifying the vaccine types most susceptible to such exposures.
Identifying biomarkers (years 1-2). Data and samples from ongoing and completed trials will be used to explore mechanisms and identify biomarkers of adverse biological status with respect to vaccine responses. In Uganda, three POPVAC trials are investigating the effects of rural versus urban location, and Schistosoma mansoni and malaria exposure and treatment, on responses to live parenteral (BCG, Yellow Fever), live oral (Typhoid [Ty21a]), Human Papilloma Virus (HPV) and toxoid (tetanus/diphtheria) vaccines; and the effect of BCG revaccination on response to other vaccines27–30. Results are expected in 2023, and samples will be available for investigation. From Kenya, data and samples are expected from malaria and other vaccine trials in various settings. Co-infection, immunological activation, nutrition, and metabolic processes will be important targets. Regarding infections, POPVAC results will guide further investigation on parasites, with a plan to explore multiplex serological analysis for exposure to viruses. High-dimensional immunological profiling of pre-vaccination POPVAC samples using CyToF and Cytek-Aurora will be undertaken: the findings, together with the Bowyer et al. results2, will inform the selection of immunological biomarkers. The focus of preparatory phase laboratory work will therefore be on nutritional markers and metabolomics using stored samples. Data emerging from work on iron and vitamin D in studies on malaria and pneumococcal vaccines will also offer further guidance. Recent data strongly suggest a key role for metabolic processes in linking nutritional and immunological parameters, with diet linked to urban-rural differences in circulating metabolites and inflammation in Tanzania31, and pre-immunisation metabolic profile linked to vaccine response32. Further exploration of metabolite analyses will be undertaken to link findings to nutritional & immunological profiles using data integration platforms such as “mixOmics”33.
Literature review. A systematic review with a primary objective of gathering existing evidence on the effects of malaria on vaccine responses in animals and humans is being undertaken. This systematic review is being conducted following a detailed full protocol that has been registered with the International Prospective Register of Systematic Reviews (PROSPERO), registration ID: CRD42022298053. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022298053
Input to the VAnguard Community Study (WP4). Based on the above, the WP1 team will guide the strategy for WP4 on sample collection and undertake laboratory investigations and analyses. This will inform the collection of whole blood (for simple cellular phenotyping, detection of active infections such as malaria or viruses [PCR or sequencing], and for [future] genetic and epigenetic analysis); and plasma (suitable for vaccine-specific, viral and parasite serology, and nutritional assessments) from all participants. Sampling for peripheral blood mononuclear cells or transcriptomics will be considered for a subset of participants.
In addition, WP1 biologists will work with the social science and engagement teams in activities to exchange and explore the understanding of vaccine biology with VAnguard communities.
Biological factors have a bearing on vaccine responses only if people are vaccinated. Both access and uptake remain an important public health problem in many LMICs, albeit with gradual improvements over the years34–37. This has been associated with many factors, including but not limited to structural, social and behavioural factors38–43.
WP2 will thus address Objective 2, to “understand how structural, social and biological determinants of vaccine response interrelate to determine vaccine impact”.
The interactions between social context, health status and vaccine efficacy are not fully understood. WP2 will investigate the relationships between specific biological vulnerabilities and broad health status, and structural and social vulnerabilities in communities will be undertaken, refining the conceptual framework to describe these interactions. While vaccine efficacy relies on biological processes, effectiveness is mediated by uptake, moderated by the willingness to participate (trust in the provider) and access. We will investigate the contribution of different information sources to knowledge and perceptions of vaccines, factors influencing vaccine availability and accessibility including geopolitical context.
Our approach will involve qualitative case studies and exploratory mixed methods44 to gain in-depth understanding of the above mentioned factors. These methods will be complemented by document reviews, interviews (individual and group), questionnaires (with WP4) and reflexive community meetings (with WP5) to provide a broader view of relevant issues in selected communities (Table 1). Work will be undertaken in both Uganda and Kenya, taking into account the social and contextual differences within and between sites.
In the preparatory phase (year 1) literature review will be undertaken to understand the relationships between the biological and structural vulnerabilities and vaccine uptake in LMIC. A systematic search guided by a search strategy will be undertaken in number of databases such as PubMed, Web of Science and Google Scholar to identify papers on the effect of communication, community engagement on vaccine uptake. All studies (both qualitative and quantitative designs) covering the above research areas will be considered excluding only review articles. Qualitative case studies will be initiated in two purposively selected communities, one each in Kenya and Uganda, and each with a specific biological vulnerability (based on infections and nutrition) identified in WP1. Case study work will proceed through years 1 and 2, to provide a holistic, in-depth understanding of how inter-relationships between structural, social and biological drivers of vaccine uptake and efficacy can combine as determinants of vaccine impact.
Findings from literature and case studies during year 1 will inform the development of questionnaires and qualitative work in the VAnguard Community Study (WP4, year 2); later findings will inform the modelling framework (WP3) and co-design of intervention packages (WP3, WP5, years 3-4).
Biological factors have a bearing on vaccine responses only if people are vaccinated. Both access and uptake remain an important public health problem in many LMICs, albeit with gradual improvements over the years34–37. This has been associated with many factors, including but not limited to structural, social and behavioural factors38–43.
Sampling: Sample sizes for qualitative work will not be decided a priori but will be dependent upon an on-going assessment of what is being learnt. Participants will be sampled purposively, ensuring maximum variation in views and key characteristics relevant to this study. Community respondents will be selected to encompass differences in gender, age, education, social-economic status, vicinity to the health facility, information access, and vaccine uptake. Data collection will end when the study team agree that no new findings are emerging, termed ‘data saturation’45. Multi-level consent processes will be applied: families will only be included if there is permission from household heads and consent from all adults. Appropriate compensation for time will be provided guided by community consultations (WP5) and country-specific guidance.
Data analysis. All data collected will contribute towards improving the understanding of relationships between structural, social and biological vulnerability and vaccine impact. Qualitative data will be uploaded into NVivo and analysed by the Framework Analysis method, a form of thematic analysis recommended for multidisciplinary research teams46. The themes derived from coding the data will be organised around major structural, social and biological vulnerabilities based initially on the conceptual framework (Figure 1), and then on findings from the initial case studies and scoping review, using the structural violence framework9. The scoping review will be done using PRISMA extension for scoping review guidelines47. The result will be a set of structural, social and biological themes that constrain decision-making, frame choices, and limit options for participants around access to, uptake of and response to vaccines. These major themes will then be further operationalised to identify and explain relevant intersections within them using the intersectionality lens14. Quantitative data will be analysed using STATA, and key findings summarised and described in tables and graphically.
WP 3 will work with WP1, 2, 4 and 5, synthesising data and leading analyses to address Objective 3, “to develop, model and test community-based integrated strategies to optimise vaccine access, uptake and effectiveness among vulnerable populations.”
The workflow is shown in Figure 3. The primary work package goal is to establish a modelling framework that describes how communities benefit from vaccine programmes and informs the development of interventions to optimise vaccine impact.
In the preparatory phase (year 1), a framework will be designed to enable data from different sources to be integrated into a coherent pathway. In this design phase, the WP3 team will participate in the literature reviews led by WP1 and WP2 which will inform parameter estimates for the model, such as disease burden and economic costs. In addition, WP3 will work with WP1 and WP2 on analyses from their initial studies, to support the design of the framework. The draft framework will guide the development of VAnguard Community Study (WP4) data collection tools, identifying areas of focus for questionnaires and sampling, and information required for economic analyses (WP4).
In the second phase, analysis and data integration from the VAnguard Community Study (WP4, year 2) will be done. Data will be summarised from questionnaires and from biological samples both overall and separately by community. Comparison will be done of communities based on their perceived vulnerability with respect to vaccine impact, their rural versus urban status, and by country, initially in crude analyses to understand where the main differences lie. Further analyses will use regression to adjust for potential confounders to determine characteristics that differ between communities after controlling for more distal factors. Further work will be undertaken to investigate interactions and undertake causal mediation analysis to assess potential mediators of more distal geographical and political factors on factors likely to be most closely related to vaccine impact (such as uptake and proxy biological profile of vaccine uptake and immunogenicity). All analyses will allow for clustering where necessary. The results from the VAnguard Community Study will be fed back into the modelling framework, to refine the model.
In the third phase (year 3) work with communities to identify and model in silico a variety of intervention packages will be undertaken. Building on the VAnguard Community Study results and modelling framework, WP3 will identify which potential determinants of vaccine impact are feasibly modifiable and apply the model to investigate the package of interventions that would optimise vaccine impact. We envisage that such packages will include integrated elements that are both biological and social. A health economics analysis will also be integrated as such interventions must be affordable and cost-effective. WP3 analyses will take the perspective of the health provider. This framework will be a unique contribution, allowing a holistic view of the overall national vaccine programmes in Uganda and Kenya as well as comprehensively considering structural, social and biological factors that can be modified to improve vaccine impact. It will also be possible to tailor the model to specific characteristics of individual communities, including demography, disease burden, context, behaviour and outcomes (including but not limited to lives saved, DALYs averted and cost per DALY averted), so that potential interventions can be considered at both national and community level. Special consideration will be given to appropriately capturing uncertainty in both model input parameters and outcomes.
Working closely with WP5 team members, the interventions designed in silico will be presented to stakeholders and communities to assess their feasibility and acceptability, and their feedback will be used in an iterative process to further refine the model and co-design intervention packages.
If funds allow, the project will carry out pilot studies to assess the feasibility of the intervention packages, and to develop and refine components of the study methods. During this pilot implementation phase, the project will closely monitor interventions by holding feedback sessions to adjust content and delivery. At the end of the piloting phase and in conjunction with WP5 team members, WP2 will conduct interviews with implementers and communities to assess the acceptability and feasibility of the interventions. This will inform the selection and design of packages for further testing in future trials.
The purpose of WP4 is to plan and implement field investigations (community surveys and qualitative studies) to explore structural, social & biological determinants impairing vaccine impact. Activities under WP4 will build on hypotheses generated and refined in the preparatory phase of WP1-3 on how structural, social & biological determinants hinder vaccine impact in vulnerable communities in Uganda and Kenya. This WP will coordinate the planning and design of the study in consultation with stakeholders and working closely with selected communities, supported by WP5.
Scoping of vaccine uptake in Uganda and Kenya and impact of COVID-19 pandemic. Besides the initial activities of WP1-3, the preparatory phase will involve consultation with stakeholders identified through national-level mapping (WP5). Immunisation programme data from the MoH’s in Uganda and Kenya will be used to identify vulnerable and less vulnerable communities in relation both to vaccine uptake and to structural factors hypothesised to impact vaccine access and coverage such as location, poverty, education, gender, migration, and disability. WP4 will examine MoH SARS-CoV-2 vaccine uptake data (when applicable) and changes in the uptake of other vaccines during the COVID-19 pandemic48. This will inform the selection of up to eight study communities (e.g. two urban, two rural in each country; one considered vulnerable and one less vulnerable in each setting).
Stakeholder mapping at the district and community level. Once communities of interest have been identified, additional stakeholder mapping and engagement at the district and community level will be done together with WP5, starting with meetings the leaders.
Consultative meeting. At approximately month 9 of the programme, WP4 will coordinate a consultative meeting, to which representatives of national and community stakeholders will be invited, and where preliminary data from WP1-3 and 5 will be presented. The primary goal will be to enable the co-design of the final VAnguard Community Study protocol.
The organisation of research teams for field activities. WP4 will organise field research teams. WP4 will plan a single team of members from Uganda and Kenya, and from bioscience and social science backgrounds, working together in each community, in collaboration with community members.
Design of data collection tools. Building on initial analyses from WP1-3, biomedical scientists will contribute standard operating procedures for the collection and analysis of biological samples. Social scientists and engagement activities (WP2 and WP5) will contribute to the development of both quantitative and qualitative data collection tools, as well as the refinement of questions on social and economic factors that may influence vaccine response, for the community survey.
Initiation of community-based work. Working with local stakeholders, community meetings will be undertaken to explain project objectives and to obtain feedback on VAnguard Community Study plans by teams comprising representatives from WP2,4 and 5.
Community Survey; collection of questionnaire data. A survey will be implemented targeting appropriate ages for vaccines identified as of particular concern during the preparatory phase. Following written, informed consent the questionnaire and simple physical examination will gather information including:
Demographic and socioeconomic characteristics of participants
Vaccination history and attitudes to vaccination
Information and information sources concerning vaccination (media access, preferences, habits etc.)
Known and hypothesised structural barriers to vaccination uptake
Health, anthropometry
Parameters for economic analysis
Collection of biological samples. The survey will include the collection of samples (blood, and urine and stool). First, these will be used to measure surrogate markers of risk of impaired vaccine immunogenicity and efficacy, based on the data assembled in WP1 (which will inform decisions on the type of samples to be collected, and assays, to be conducted). Second, they will be used to provide a proxy biological profile of vaccine uptake, including tetanus toxoid antibody: tetanus immunisation is expected during infancy, with boosters at school and in pregnancy.
Sample size. 172 participants will be enrolled per community (of appropriate ages, see above) giving 80% power to detect differences (odds ratios ≥2) in exposure prevalence between vulnerable and less vulnerable communities for exposures with prevalence ≥20% and greater power for more common exposures (Table 2). Assuming that the standard deviation of continuous outcome measures (e.g. antibody responses) will lie between 0.2 and 0.6 log10, with responses in vulnerable settings 0.1 to 0.2 log10 lower than in less vulnerable settings, and allowing for ~10% of participants not giving blood samples, 172 participants from each community will give >80% power to compare responses between vulnerable and less vulnerable communities in each setting.
Shaded areas indicate odds ratios that VAnguard Community Study would have the power to detect for a range of exposure prevalence.
WP5 will collaborate with WP1-4, to achieve objective 4, “to empower vulnerable communities to optimise vaccine impact for their people through a process of co-learning and co-creation between them and researchers”. In this regard, WP 5 will contribute to the activities of WP 1-4 in terms of literature review, tools design and data analysis as appropriate. In addition, WP5 will support engagement with other stakeholders, such as policymakers, and broader public engagement.
WP5 will develop a comprehensive Communication and Engagement plan taking into account NIHR’s guidance for community engagement and involvement in global health research49 to guide the Vanguard project’s Stakeholder, Community and Public Engagement and Involvement activities.
Stakeholder mapping and network analysis. WP5 will map and engage with vaccine-related stakeholders in Uganda and Kenya (building on WHO’s comprehensive stakeholder listing50) to develop a stakeholder mapping and influencer network analysis specific to Vanguard’s goals of improving vaccine impact for vulnerable people. This will involve consultative meetings in each country, starting with (i) national-level stakeholders from the MoH’s, national vaccine programmes and global vaccine groups (GAVI, UNICEF, WHO), (ii) District/County Health Managers, (iii) Community leaders and representatives at the local site level, (iv) religious leaders. To ensure coordinated public information and interventions, key actors will be included in all stakeholder engagement activities.
Co-designing the VAnguard Community Study (WP4). National and district/county-level stakeholders will assist in the identification of vulnerable communities for engagement. Community representatives will confirm identified communities as a true reflection of vulnerability and add any that may have been missed by national stakeholders. The process will lead to a shared decision regarding communities to be included in the VAnguard Community Study.
WP5 will work with the selected communities to identify individuals and networks relevant to successful vaccination implementation interventions (such as health-rights activists, front-line workers, local leaders, religious leaders, and civil society actors). These will be engaged to understand power dynamics and socio-structural barriers to vaccination uptake. Local government officials, community and religious leaders will be consulted at inception to ensure the relevance and quality of the research approaches and tools, and throughout the project to facilitate reflexivity and secure buy-in.
The methodology will include interpersonal channels, consultative meetings involving District/County Health Managers, community representatives at the local site level, religious leaders and other civil society actors and influential personalities to be identified iteratively in the communities. Views from these meetings will inform aspects of the VAnguard Community Study design, including collaboration with WP2 and WP4 in the development of “knowledge, attitudes and practice” (KAP) elements of the study questionnaire. Communities will also be involved (including through pre-testing) in validating the research agenda and checking cultural relevance of language and approach. The community will be engaged throughout the project via participatory radio programmes and regular progress updates through co-identified appropriate communication channels.
Enhancing researcher knowledge of communities’ understanding of vaccines, vaccine needs, gender perspectives regarding vaccines, and the impact of media, including social media on vaccine uptake. In collaboration with WP2, WP5 will hold eight reflexive meetings (one in each selected community) using participatory methodologies with stakeholders and community representatives and identified vulnerable groups, to understand perspectives on vaccines, including factors that hinder or facilitate vaccine uptake (structural, social, biological).
A particular area of focus in the VAnguard project is to understand how people obtain information about vaccines and what information sources they use and trust. Building on the outcome of the community survey, WP5 will hold media workshops in Kenya and Uganda, targeting national and local media players to discuss the impact of media content, including social media, on vaccine attitudes.
Co-design strategies to optimise vaccine uptake among the communities. WP5 will collaborate with WP3 (and other WPs) to bring together all stakeholders/communities in one workshop to share the results of data analyses and contribute to the co-design of vaccine interventions.
Broader public engagement. The following activities will be undertaken to engage the broader population and provide platforms for them to share their views on the project. Where relevant, WP5 shall engage and collaborate with other NIHR GH groups and units interested in vaccines, and wider, African, vaccine development and implementation networks in these activities.
Community meetings in VAnguard Community Study areas.
Radio shows
Community drama
Quarterly webinars to update the public about research progress, and one final one coinciding with the final dissemination; the webinars will involve trusted sources such as government officials, academics, and community leaders, in conjunction with the research team.
Work with MoH’s, researchers, community members, journalists and influencers to develop accurate articles and messages about vaccines.
Development of contextualised short videos (or other short-form media messages) to share vaccine experiences and relevant information and updates.
An effort will be made to render every message for the public in multi-media and multi-lingual format.
WP6 addresses objective 5: “to build capacity for, and a culture of, consultative, collaborative multidisciplinary vaccine research in East Africa”focusing on areas as outlined below.
Strengthening institutional research support structures. VAnguard will fund research support positions that require development, for example in communications, M&E, and work to ensure that the positions are institutionalised for sustainability after the end of the project.
Research support training. WP6 will provide training for both researchers and research support staff among VAnguard’s partner institutions to promote quality research management. WP6 will adopt a two-pronged approach involving mentoring and coaching from experienced colleagues and continuous professional development courses. To facilitate networking with other managers, WP6 will support institutional subscriptions to the Eastern Africa Research and Innovation Management Association (EARIMA), which promotes members’ professional development and capacity building, and best practices in research management and administration.
Strengthen grant management among partner institutions. To standardise and strengthen grant governance, and increase funders’ confidence in grants management, Group institutions will be supported to achieve Good Financial Grant’s Practice (GFGP) accreditation. This will include supporting managers to draft policies and guidelines, providing enhanced grant management software (upgrading from QuickBooks to Navision at UVRI) and building institutional systems.
Developing leadership skills among VAnguard early-mid career (EMC) researchers. Postdoctoral researchers will be the engines driving research in the VAnguard project. To gain experience, they will take on leadership roles including leading the scientific WP (detailed above) and supervising PhD students. They will be mentored formally and informally by senior researchers from VAnguard partners and collaborating institutions to implement career development and training plans. This will hone their skills to become future research leaders.
Generic and regulatory skills training for VAnguard researchers and research managers. WP6 will establish joint trainings to improve the professional and technical skills of both researchers and research managers. These will focus on scientific writing, grant writing, science journalism, policy analysis, media, public and community engagement, evidence appraisal, scientific preparedness for emergencies and research management. In addition, WP6 will support and ensure training required for good practice, including the responsible conduct of research and safeguarding (with WP5), Good Clinical Research Practice (GCP) and Human Subjects Protection (HSP).
PhD training WP6 shall support three PhD students registered in local universities (Makerere University, Uganda Christian University) or the Open University (OU) if based at KWTRP, which is an affiliated research Centre of the OU). The studentships will be awarded competitively, with attention to balance between WP, institutions and countries, as well as diversity and inclusion. The PhD projects will be embedded within the WP and students will be supervised by the EMC scientists, as part of promoting research leadership, with academic supervisors in the universities. Students will also benefit from co-supervision by professors from partner universities in the UK.
Vaccine-related statistical modelling course. A modelling workshop will be organised with WP3, focussing on modelling vaccine-related data. The course will benefit from the datasets that have been gathered among partner institutions, and from the VAnguard Community Study
Evidence synthesis and policy engagement training. WP6 shall train researchers to understand how vaccine rollout policies are developed, how to package their output in a suitable format for policymakers and how to gain buy-in from health stakeholders.
Developing vaccine research capacity in other states
VAnguard plans to “foster” four MSc students from the University of Makeni in Sierra Leone (UNIMAK), studying relevant topics (e.g. immunology and clinical microbiology, biostatistics, and social science) in Uganda, to undertake projects within the VAnguard programme. UNIMAK will provide travel, fees, and upkeep; VAnguard will provide mentorship, supervision, and project costs within the research programme, and will involve the students in professional and technical skills training. At the end of the course, the students will be supported to apply for scholarships to support their next level of training. The integration of MSc students from Sierra Leone in VAnguard will build capacity and facilitate East-West Africa partnerships as a platform for future vaccine research activities.
VAnguard project will be guided by an Advisory Group and led by a Steering Committee (SC) comprising all study investigators as well as community representatives.
The Advisory Group (AG) will meet annually, in conjunction with VAnguard Annual Group Meetings. The AG will comprise experts in key areas of interest: biological science; social science and community engagement and vaccine policy implementation, as well as leaders of related vaccine initiatives.
The SC will meet twice in the first six months, then 6-monthly. The day-to-day work of the project will be managed by an executive committee. The executive committee will comprise all WP leads, and administrative, M&E and communication leads, and will meet quarterly (more frequent meetings may be required at the start). Work package teams will meet monthly, as will the administrative team.
Project outcomes are.
1. Outcome 1: Knowledge and understanding of the interacting structural, social and biological determinants of population and community differences in vaccine impact
2. Outcome 2: Evidence-based, integrated strategies to optimise vaccine effectiveness for vulnerable communities.
3. Outcome 3: Strengthened capacity for multidisciplinary vaccine research, characterised by a culture of multidisciplinary collaboration, and co-creation with stakeholders and communities, facilitating pathways to the desired impact: improved community health and reduced health inequity.
The study is in the preparatory stages of year 1. Analysis of stored samples by WP1 to identify biomarkers is underway. The WP1 protocol of the systematic review on effect of malaria on vaccine responses has been published by Prospero and is underway. Plans are underway to undertake the literature review and case studies in WP2. Currently the protocol of the case studies has been submitted to research and ethics committees in Kenya and Uganda for review and approval. Process has started to draft the WP4 protocol for the year two community survey.
Ethical review and approval will be sought from the UVRI Research Ethics Committee and Uganda National Council for Science and Technology; the KEMRI Scientific Ethical Review Unit; and the ethics committee of the London School of Hygiene & Tropical Medicine.
Inclusion of minors: parental consent will be required, with assent from children above the designated age in each country; emancipated minors will be able to give their consent.
Illiteracy, or unfamiliarity with languages in which information and consent forms are provided: independent witnesses able to speak both relevant languages will be invited to help
Management of illnesses identified incidentally and requiring treatment: results will be provided to the participant; counselling and treatment will be provided, or appropriate referrals made.
Permission for, and use of, stored samples: separate written informed consent will be requested for use of samples obtained for future studies. For WP1 work on existing samples or data, evidence will be required that participants consented to such use; approval for the studies will be sought from relevant committees unless covered by existing approvals.
To ensure compliance with international standards, GCP and HSP training will be provided, and staff will be required to hold an up-to-date certification. In addition, the study will be monitored with site initiation, interim and close-out visits for compliance, quality assurance and training purposes51.
Pathways to the desired outcome of improved vaccine impact, and the ultimate goal of better health for vulnerable communities and reduced health inequity will include:
Engagement with stakeholders and communities and co-production of outputs (WP2,4,5)
Knowledge translation and dissemination of findings to broader stakeholder groups including academics, implementers, politicians, research communities, and the public through local, national, regional and international meetings, research papers, reports (WP1-4), research networks, websites, policy briefs, blogs, social and conventional media (WP5). We shall publish in Open Access journals and make our data accessible for wider use.
Further research is to test the selected interventions, harnessing mentorship and building the capacity of African scientists to lead new proposals building on this work (WP6).
The Group will reach out to colleagues involved in related programmes across the region and in other NIHR programmes to develop networks, for example by sharing opportunities to participate at each other’s meetings and on each other’s Advisory Boards.
Uganda and Kenya are well placed to host this research project. VAnguard researchers from Uganda are currently running three trials investigating the role of parasites and other infections in vaccine immunogenicity (POPVAC)27, collecting samples that will contribute to the proposed work. Kenyan VAnguard researchers are exploring the role of nutritional factors affecting vaccine responses in Kenya. Identifying modifiable mechanisms would enable the optimisation of vaccine efficacy.
Held jointly at the UVRI, Uganda and the KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya, the three-day launch event brought together stakeholders in vaccine research in discussions to understand the project, share knowledge and ideas and contribute to the evidence base of factors that influence vaccine impact for vulnerable communities.
Meeting participants included members of the Scientific Advisory Board, Steering Committee, Executive committee, partners, collaborators, student supervisors, The Makerere University – Uganda Virus Research Institute (UVRI) Centre of Excellence for Infection & Immunity Research and Training (MUII-plus) Centre members & alumni, PhD, postdoc fellows and representatives of the communities and Ministry of Health officials from both countries. The event was structured to include presentations from representatives of NIHR, UVRI and KEMRI-Wellcome Trust vaccine research. Keynote speeches were delivered by experts in the areas of bioscience, social sciences, and community engagement on day 1. On the second and third day discussions were held in respective WP to better understand the planned activities and plans to inform implementation.
Prof Pontiano Kaleebu (VAnguard Co-Lead and Director UVRI) gave an overview of vaccine research at the Institute. He noted that this research spans the different stages of vaccine development, including social science, epidemiology, and laboratory sciences; design and manufacture of vaccines, Phase I/II trials (Ebola, Rift Valley, COVID-19 vaccines), large-scale Phase II trials and post-licensure that involved potency testing (Measles/Polio) and dose optimization (Yellow fever). Dr Sarah Puddicombe, the Assistant Director Global Health, NETSCC, gave a talk on how the VAnguard project is closely aligned with the NIHR aims and strategic plans. Funded by the Department of Health and Social Care, NIHR was established in 2006 as part of the UK strategy Best Research for Best Health, with a mission to improve the health and wealth of the UK through research. In 2016, NIHR also became a major funder of applied health research in low- and middle-income countries using Official Development Assistance budget (known as UK Aid) from the UK Government. Dr. Alfred Driwale (Program Manager- MOH/UNEPI) gave a presentation on the immunization programme in Uganda highlighting issues and interventions. He highlighted the fact that the trend of DPT coverage, an indicator of immunization uptake has steadily improved over the past 42 years. From the Kenyan perspective, Dr Lucy Mecca (Head, National Vaccines and Immunization Programme, Ministry of Health, Kenya) and Christine Mataza from Kilifi County Department of Health shared an update on the challenges and successes of the vaccination programme in Kenya.
The keynote speaker of the biological factors session, Prof Maria Yazdanbakhsh (Leiden University Medical Center) described how different immunological footprints resulted from different environmental exposures. Her group’s research findings suggest that future vaccine research studies should be conducted in populations from different parts of the world to provide a more complete picture of the immune system where these vaccines may be deployed. In another stimulating keynote talk, Mr. Robert Kanwagi (London School of Hygiene & Tropical Medicine) discussed experiences and lessons learnt from Ebola and COVID-19 vaccine deployment in Africa. From this talk, he showed that vaccine deployment strategies in Africa should be locally informed to improve vaccine uptake; that is, they should be context-specific, consider the local vaccine supply and demand, incorporate multicomponent planning at all stakeholder levels, and establish cross-sectoral partnerships.
Dr David Mafigiri (Makerere University) discussed how social science contributed to vaccine trials research, such as by improving the understanding of the political and economic context of the trials, community perceptions, concerns and expectations, and ethical issues surrounding clinical trials. Dr Annette Kezaabu (Uganda Christian University) discussed the role of community engagement and involvement (CEI) in vaccine research in Koome fishing villages in Uganda. Finally, Noni Mumba (KWTRP), shared experiences from community engagement (CE) activities in vaccine studies conducted at KWTRP in Kilifi, Kenya.
On day two and day three, two breakout sessions were conducted, one on choosing communities to work in and the second one on work package planning.
Key recommendations from the meeting, focusing on choosing the communities to work in included:
1. A clear definition of what the project means by vaccine vulnerability should be finalized at the beginning of the project, the team proposed the consideration of factors such as: vaccine uptake, hard-to-reach, rural-urban setting, and type of environment.
2. Need to carefully define what constitutes a 'community' - is it in terms of village, county/district?
3. Develop an engagement plan for approaching communities that may be hesitant to participate in health research.
4. Consider communities that current investigators have been working in, as data on the suitability of the populations are available.
5. Map all available samples collected from vaccine studies at UVRI and KEMRI-Wellcome Trust Research Programme.
6. Consider looking at available samples and using these to inform selection of communities. Considerations could include rural and urban communities in Uganda and Kenya with differing prevalence of parasitic infections (helminths, malaria).
7. To examine the Ministry of Health's vulnerability mapping index data (the index shows which communities are vulnerable based on several factors such as access to health services, age, etc.). In addition, activities to inform the final selection of communities for the survey, such as gathering data to define vulnerability, engaging with stakeholders, and collecting relevant data such as vaccine uptake.
VAnguard will bring together African and UK experts in vaccine research, implementation and stakeholder and community engagement, to work in Uganda and Kenya.
Results from this study will identify modifiable structural, social and biological determinants of impaired vaccine impact in vulnerable African communities. From this, integrated strategies will be developed to drive health equity. This project will allow researchers, relevant stakeholders, and the community to contribute to investigating in detail which biological and social factors most influence vaccine impact in vulnerable communities.
Ensuring that the results of our study are incorporated into relevant guidelines and policy statements is important. This will be achieved through working with relevant stakeholders and the community, and also through publishing the findings in peer-review journals.
We would like to thank all project team members and contributors to the VAnguard programme for their valuable input to date: Alison Elliott (London School of Hygiene and Tropical Medicine), Pontiano Kaleebu (Uganda Virus Research Institute), Sarah Atkinson (KEMRI-Wellcome Trust Research Programme), Katie Ewer (University of Oxford), David Mafigiri (Makerere University), Primus Chi (KEMRI-Wellcome Trust Research Programme), Dorcas Kamuya (KEMRI-Wellcome Trust Research Programme), Heidi Larson (London School of Hygiene and Tropical Medicine), Agnes Natukunda (MRC/UVRI & LSHTM Uganda Research Unit), Emily Webb (London School of Hygiene and Tropical Medicine), Caroline L Trotter (University of Cambridge), Ludoviko Zirimenya (London School of Hygiene and Tropical Medicine), Monica B Chibita (Uganda Christian University), Noni Mumba (KEMRI-Wellcome Trust Research Programme), Henry Luzze (Ministry of Health, Uganda), Achilles Kiwanuka (MRC/UVRI & LSHTM Uganda Research Unit), Samson Kinyanjui Muchina (KEMRI-Wellcome Trust Research Programme), Eleanor Martins (London School of Hygiene and Tropical Medicine), Moses Kizza (Uganda Virus Research Institute), Raymond Muganyizi (Uganda Virus Research Institute), Gyaviira Nkurunungi (London School of Hygiene and Tropical Medicine), Victoria Bukirwa (Uganda Virus Research Institute), Reagan Mogire (KEMRI-Wellcome Trust Research Programme), Dorcas Mbuvi (KEMRI-Wellcome Trust Research Programme), Annette Kezaabu (Uganda Christian University), Henry K Karanja (KEMRI-Wellcome Trust Research Programme), Flavia Zalwango (Uganda Virus Research Institute), Esther Owino (KEMRI-Wellcome Trust Research Programme), Ronald Nkangi (Uganda Virus Research Institute), Laureen Kahunde (Uganda Virus Research Institute), Ritah Namagembe (Uganda Virus Research Institute), Winnie Eoju (Uganda Virus Research Institute), Robinah Nalwanga (MRC/UVRI & LSHTM Uganda Research Unit), Robert Kanwagi (London School of Hygiene and Tropical Medicine), Alex Hinga (KEMRI-Wellcome Trust Research Programme), Caroline Jones (KEMRI-Wellcome Trust Research Programme).

Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
No
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious Diseases
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious diseases; vaccinology; public health; disease prevention; health systems research
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 26 Feb 24 |
read | read |
|
Version 1 27 Jun 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)