Keywords
Hypertension, Kenya, The Gambia, Blood pressure, Hypertension mediated organ damage, Community
Sub-Saharan Africa (SSA) has one of the highest prevalences of hypertension worldwide. The impact of hypertension is of particular concern in rural SSA, where access to clinics and hospitals is limited. Improvements in the management of people with hypertension in rural SSA could be achieved by sharing diagnosis and care tasks between the clinic and the community. To develop such a community-centred programme we need optimal approaches to identify and risk stratify patients with elevated blood pressure. The aim of the study is to improve the evidence base for diagnosis and risk estimation for a community-centred hypertension programme in two rural settings in SSA.
We will conduct a cross-sectional study of 1250 adult participants in Kilifi, Kenya and Kiang West, The Gambia. The study has five objectives which will determine the: (1) accuracy of three blood pressure (BP) measurement methods performed by community health workers in identifying people with hypertension in rural SSA, compared to the reference standard method; (2) relationship between systolic BP and cardiovascular risk factors; (3) prevalence of hypertension-mediated organ damage (HMOD); (4) accuracy of innovative point-of-care (POC) technologies to identify patients with HMOD; and (5) cost-effectiveness of different combinations of BP and HMOD measurements for directing hypertension treatment initiation.
This study will determine the accuracy of three methods for community BP measurement and POC technologies for HMOD assessment. Using the optimal methods in this setting it will estimate the prevalence of hypertension and provide the best estimate to date of HMOD prevalence in SSA populations. The cost-effectiveness of decision-making approaches for initiating treatment of hypertension will be modelled. These results will inform the development of a community-centred programme to improve care for hypertensive patients living in rural SSA. Existing community engagement networks will be used to disseminated within the research setting.
Many people live with high blood pressure in sub-Saharan Africa. In this region, the proportion of people with high blood pressure is one of the highest in the world. However, few people with high blood pressure are treated and this can lead to serious medical issues and even death. This is particularly true in rural areas where treatment and understanding of blood pressure is lower than in cities.
There are many reasons why high blood pressure is a major health problem in rural sub-Saharan Africa, such as a lack of clear symptoms; less access to healthcare; and limited time to travel to clinics for care. One option for improving the management of blood pressure is to use a community-centred approach, where care is brought into the community making it easier to access.
To bring care into the community, we need to find out what is the best way for community health workers to identify who needs to be treated. Standard techniques may not be useful in a rural community and could require too many resources to make them practical. This study aims to determine what is the best way to identify high blood pressure and related health complications in a community setting.
The study will take place across two sites: one in Kilifi, Kenya and the other in Kiang West, The Gambia. We will enrol 1250 participants, with 625 in each country. The people living in these areas have been involved in the design of this study through community engagement and have helped identify the need for improving how blood pressure is treated in a rural areas. Throughout this study, we will continue to meet with the communities. Once the study is completed, we will use our strong links with the communities, healthcare providers and policymakers to share the results.
Hypertension, Kenya, The Gambia, Blood pressure, Hypertension mediated organ damage, Community
This new version contains the following changes in response to peer review comments:
See the authors' detailed response to the review by Liangdi Xie
See the authors' detailed response to the review by Destaw Fetene Teshome
Hypertension is the single risk factor that accounts for the highest number of deaths (10.8 million) globally1,2. Sub-Saharan Africa (SSA) has one of the highest estimated prevalences of hypertension worldwide, and in 2019 hypertension was implicated in almost 700,000 deaths in SSA – double the number in 19901,2. In SSA, hypertension occurs at younger ages, is more severe, remains very poorly controlled, and is more likely to cause complications including heart failure, stroke, kidney disease and premature death than in other regions3,4.
The impact of hypertension is of particular concern in rural SSA where 60% of the region’s 1.1 billion population live. While hypertension prevalence is as high in rural as in urban settings, hypertension awareness, treatment and control is lower in rural settings5–7.
The NIHR Global Research Group on Improving Hypertension Control in Rural Sub-Saharan Africa (IHCoR-Africa) has been set-up to address these pressing issues and the study described here is part of that effort.
To significantly reduce hypertension-related burden, there is a need to improve the whole hypertension control cascade from awareness (screening and diagnosis) to management (risk stratification and treatment) and control (monitoring, adherence, and referral)8. The barriers to effective hypertension management in rural SSA are multiple and complex. At an individual-level, key issues are the asymptomatic nature of hypertension, a lack of understanding of long-term risk, financial impact of taking time away from work to seek treatment, caring responsibilities, and misconceptions about the benefits of pharmocotherapy9–13. At the provider-level, barriers include poor communication between providers and patients, lack of skills and competencies, poor infrastructure, and lack of adequate referral systems for care14. Finally, system level barriers include poor access to health care facilities (caused by overcrowding and long distances between homes and clinics); irregular access to medications; lack of affordable treatments including anti-hypertensive drugs; and underinvestment in health service capacity15.
There is growing evidence from other regions where access to medical care is limited that community health workers (CHWs) can play a key role in managing hypertension and its consequences16–18. The HOPE-4 study, conducted in Colombia and Malaysia, showed that a community-centred intervention including screening, diagnosis and treatment with combination pharmacotherapy administered by CHWs and supported by electronic decision support tools improved blood pressure control19. Similarly, the COBRA study, in Southeast Asia also showed improved hypertension management delivered using a community-centred intervention by CHWs20. This evidence base has led organizations such as the Pan African Society of Cardiology (PASCAR) to advocate for community-centred approaches to improve hypertension management in SSA21.
However, equivalent studies in rural SSA are lacking, resulting in limited evidence for clinical recommendations in these settings. Specifically, it is unclear whether recommended approaches to the diagnosis of hypertension and estimation of cardiovascular disease (CVD) risk are appropriate and effective for use in these settings. Clinical guidelines, developed from high-income country studies, ubiquitously incorporate these two aspects when guiding decision making on treatment, including pharmacotherapy22,23. International guidelines recommend repeated clinic-based blood pressure (BP) measurement such as Automated BP Measurement (ABPM), which can be performed with (attended; aABPM) or without (unattended; uABPM) a health worker present, to mitigate for white coat hypertension. Home Blood Pressure Measurement (HBPM) or 24-hour ambulatory blood pressure monitoring (24-hr ABPM) are also deemed to have a role (Table 1). However, these international recommendations might not be accurate, feasible or cost-effective in SSA and require further investigation24,25.
International guidelines use an absolute risk approach to inform treatment. For example, although all guidelines recommend starting anti-hypertensives for a person with a BP over 160/100 mmHg, the International Society of Hypertension guideline only recommends anti-hypertensives for someone with a BP over 140/90 mmHg, when they are at high CVD risk as determined by risk scoring26. In rural SSA, the suitability of existing risk scores is unclear. First, risk scores were developed from populations where the prevalence of known risks such as smoking and high cholesterol is much higher than in rural SSA27–29. Second, compared to high-income countries, most studies in SSA show a substantial burden of hypertension on younger age groups but the age-component of current risk scores classify younger individuals as at minimal risk. This is particularly pertinent given that age-adjusted cardiovascular event rates are higher in low- and middle-income countries than in high-income settings30–32. Third, current risk scores do not consider region-specific factors in SSA, including co-existing chronic communicable disease (HIV and malaria), or exposure to environmental factors (indoor air pollution)33–35. Finally, genetic factors could be contributing to the different epidemiological picture of hypertension and CVD risk in SSA36,37. Therefore, novel approaches to risk estimation are required.
One strong candidate that may have a key role in optimising risk stratification in rural SSA is hypertension-mediated organ damage (HMOD), which includes subclinical damage to organs including the heart, eyes, and kidney among other organs. HMOD has been reported to occur at younger ages and be more prevalent in SSA population38,39. Assessment of HMOD is recommended by clinical guidelines and is strongly associated with CVD among people with hypertension22,23,26. However, significant challenges persist to measuring HMOD in routine practice, including the clinical resources required for echocardiograms, electrocardiograms, retinal imaging and laboratory blood analysis. Novel point-of-care (POC) diagnostic tools may remove this barrier but have not been validated in this setting.
A key unaddressed challenge is how to embed any new techniques for BP and HMOD measurement within the local health system, especially when CHWs are given more responsibilities without a parallel increase in resources and clinical supervision. To be feasible and sustainable, a community-centred approach to managing hypertension must take account of the health system context.
This paper reports the design of one study that is part of the wider NIHR IHCoR-Africa Group. IHCoR-Africa will also conduct another study addressing the health system challenges and patient experiences will be reported separately. The findings from both studies will contribute to the development of a new community-based intervention for improving hypertension management in rural SSA.
This study has been developed through an equitable collaborative partnership between the London School of Hygiene & Tropical Medicine (LSHTM; UK); the Kemri-Wellcome Trust Research Programme (KWTRP; Kenya) and the MRC Unit the Gambia (MRCG; the Gambia). This partnership was formed during the proposal phase and involved preparatory public and participant involvement and engagement to define the research objectives. Protocol drafting was supported by weekly meetings with representatives from all three partners and included two in person development meetings: November 2022 in Kilifi, Kenya and February 2023 in Fajara, the Gambia. The structure of the partnership enabled all members to have meaningful input in the design of the study.
This study has a strong emphasis on the involvement of the patients and the public. Before protocol development, the study was discussed with community leaders in the study areas so they could suggest changes and discuss how to involve the community. The IHCoR-Africa project has been presented to local departments of health in Kilifi and Gambia, and their recommendations taken into consideration. Throughout the course of the study we will meet with CHWs and patient groups, to understand their experiences as the project is implemented. The collection of data will be mainly performed by CHWs, who are members of the studied communities. After the conclusion of the study, we will involve CHWs and other members of the community in developing an intervention that should improve the detection, treatment, and control of hypertension in rural Gambia and Kenya.
Activities will take place across two rural sites, one in East Africa (Figure 1). Both settings have a high burden of hypertension, as well as a well-developed research infrastructure to facilitate our work.
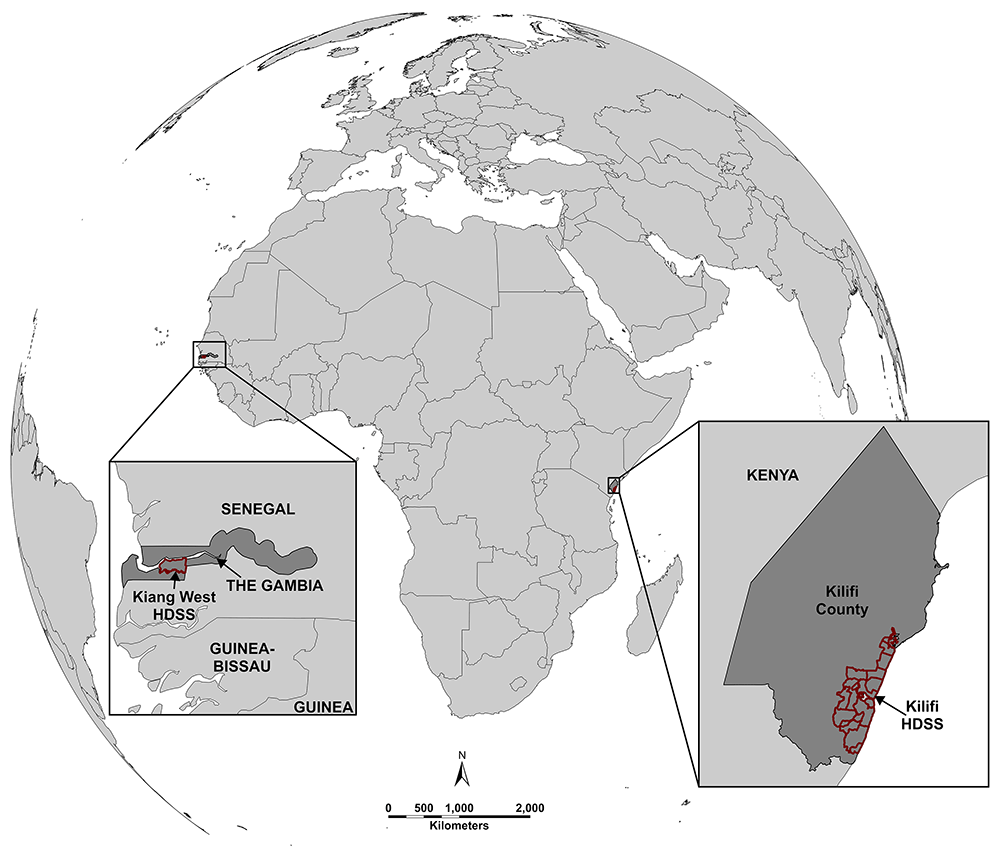
West Kiang Health and Demographic Surveillance System (The Gambia) and Kilifi Health and Demographic Surveillance System (Kenya). This figure is an original figure produced by the authors for this article.
Kilifi County is one of the poorest regions in Kenya40. The Kilifi Health and Demographic Surveillance System (KHDSS), covers an area of 900 km2 and has a population of around 300,000 people. The prevalence of hypertension is 26% and only 3% of individuals have their blood pressure controlled41. Further evidence of the consequences of poor hypertension control is the high incidence of cardiovascular disease in the area, with more than one third of patients admitted with cardiovascular disease dying during their hospital stay31.
CHWs deliver health services at the community level and work within a Community Health Unit (CHU), a health service delivery structure within a defined geographical area covering approximately 5,000 people (500–1000 households). CHWs are selected from the community they serve and where possible, are required to have completed secondary education. Each CHU is required to have approximately 10 CHWs.
The Kiang West district in The Gambia covers 750km2 and includes 36 villages with a population of over 14,000 individuals. It is one of the poorest regions in the country with all households in the lowest or low wealth groups. Hypertension prevalence in this region is estimated to be 40%, of whom at least 71% are undiagnosed, and only 4% controlled42. The health service delivery in the region is coordinated through the Regional Health Team of the Lower River Region (Mansakonko Administrative Area). The community health nurses (CHNs) deliver services in a defined village cluster within the district. There are 4 CHNs in Kiang West with each handling 7–9 villages. CHNs work with and supervise the village health workers (VHW) who are selected by their communities.
We will select an age-stratified random sample of participants aged ≥30 years from the population registers of the Kiang West Health and Demographic Surveillance System (KWHDSS, the Gambia) and Kilifi Health and Demographic Surveillance System (KHDSS, Kenya)43,44. Half of the study population will be selected from The Gambia and half from Kenya. The following age strata will be used: ≥30 to <40, ≥40 to <50, ≥50 - <60, ≥60 - <70 and ≥70y.
Pregnant women will be excluded due to technical difficulties in conducting some of the study measurements such as the echocardiogram and while gestational hypertension remains important it is beyond the scope of our aims and objectives. The participant recruitment approach is shown in Figure 2.
Inclusion criteria:
• Registered in population registers of the KWHDSS (The Gambia) or KHDSS (Kenya)
• Aged ≥30 years at the time of enrolment in the study
• Able to provide written informed consent to participate in the study
Exclusion criteria
• Pregnant women (self-reported)
We aim to improve the evidence base for diagnosis and risk estimation for a CHW-led community-centred hypertension programme in two rural communities in SSA.
We have the following specific objectives:
1. To determine the accuracy of three alternative blood pressure measurement methods relative to the reference standard and establish the prevalence of hypertension
2. To determine the relationship between systolic blood pressure and potential risk factors
3. To determine the prevalence of HMOD
4. To determine the accuracy of innovative POC technologies to identify patients with HMOD
5. To estimate the likely cost-effectiveness of different combinations of blood pressure and HMOD measurements to initiate pharmacological treatment in people with hypertension
The overall sample size for the study is based on the assessment of hypertension prevalence. Assuming a prevalence of hypertension of 25–35%, as detected by the reference standard method (24-hr-ABPM), we will require 832–1003 participants to generate validity parameters for hypertension prevalence with a precision of ±3% overall and ±5% at each of the two sites24,41. We have made a provision for loss of data in 25% of participants due to poor data quality, taking the total number of participants required to 1250, equally divided between Kilifi (N=625) and Kiang West (N=625). This overall sample size will provide sufficient statistical power to address the other aims stated above.
In our assessment of BP and risk factors, and assuming for illustrative purposes that 10% of individuals will have any of the putative factors and that the standard deviation of systolic blood pressure will be 15 mmHg, then 1,003 participants will provide at least 80% statistical power for an alpha of 0.05 to detect a 4mm Hg difference in systolic BP in those with and without the exposure of interest.
Assuming a prevalence of hypertension detected by the reference standard method (24-hr-ABPM) of 30%, we estimate that approximately 300 participants from those recruited in Objective 1 will be classified as hypertensive. With 300 hypertensive patients, we would be able to estimate the confidence interval for a conservative HMOD prevalence of 35% with lower and upper intervals of 29.7% and 40.4% respectively, and that we will have over 95% power for an alpha of 0·05 to test the null hypothesis that the prevalence of HMOD is below 25%. To further explore the difference in the prevalence of HMOD in the hypertensive and general population, we intend to assess all participants recruited into the study for HMOD.
When measured with the reference standard techniques, we expect a 35% prevalence of HMOD among the 300 estimated hypertensive participants and approximately 5% prevalence in the remaining non-hypertensive participants. This will provide us with a total available sample of 153 participants with HMOD. Assuming at least 85% sensitivity of detection of HMOD by the POC strategies we will have at least 80% power for an alpha of 0.05 to test the null hypothesis that the sensitivity is below 75%. As above, we will conduct POC HMOD assessments on all participants.
Baseline assessments. Baseline assessments will collect information including demographics, household assets, anthropometrics, diet, physical activity, history of hypertension and medication use for hypertension and other diseases (Table 3).
Blood pressure assessment. We will conduct a cross-sectional study over two weeks to determine the accuracy of three BP measurement methods compared to the reference standard of 24-hour Ambulatory BP Measurement (24-hr-ABPM). The alternative BP measurement methods are: 1) Attended Automated BP Measurements (aABPM); 2) Unattended Automated BP Measurements (uABPM); and 3) Home-Based BP Measurement (HBPM). Details for all BP measurement methods are summarised in Table 1 and Table 3.
All BP measurements will be conducted by CHWs trained by the study team to perform the blood pressure measurements based on study SOPs. The competencies of the CHWs will be assessed by the study team prior to data collection and at regular intervals during data collection. The CHWs will then collect blood pressure measurements in a random order at participants’ homes. The random order of blood pressure measurements for each of the participants will be set using a computer-generated randomisation list produced prior to measurements starting.
Each participant is expected to have completed all four BP measurements over a two-week period. A schematic overview of the procedure is shown in Figure 3. We will follow relevant international cardiological society guidelines for conducting all BP measurements45–47.
Hypertension mediated organ damage assessment. We will measure HMOD in the following organ systems: heart, eye, kidney, and vasculature. All measurements will be conducted during an in-clinic visit. The prevalence of HMOD will be assessed using reference-standard clinical assessments. In addition, we will validate the accuracy of POC assessments for HMOD compared to the reference-standard methods for the heart, eyes, and kidney. For all HMOD assessments, a detailed SOP will be used to ensure consistency between both study sites (Kenya and The Gambia) and staff will be trained in the relevant competencies. HMOD definitions in all categories will follow existing clinical guidelines22. These assessments are summarised here and described in Figure 4 and Table 2 and Table 3.
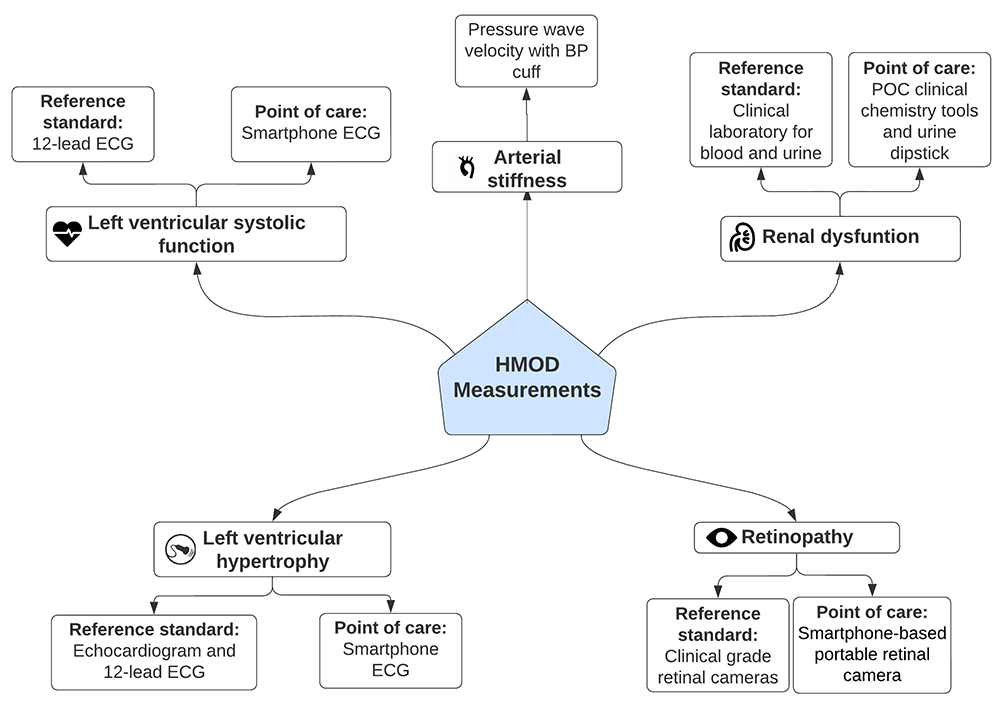
Reference standard HMOD assessment
In the heart, left ventricular systolic dysfunction will be assessed with a transthoracic echocardiogram (TTE), and left ventricular hypertrophy will be assessed with a TTE and 12-lead electrocardiogram (ECG). In the eye, hypertensive retinopathy will be assessed from retinal images captured by a fixed retinal camera and graded for hypertensive retinopathy by trained staff. Kidney dysfunction will be assessed using clinical laboratory assessment of blood and urine to measure serum creatinine levels and estimate glomerular filtration rate (eGFR) and glomerular function using urine albumin:creatinine ratio and proteinuria. Finally, we will assess pulse wave velocity and arterial stiffness using a specialised blood pressure cuff (Arteriograph, TensioMed, Hungary).
Point-of-care HMOD assessment
Point-of-care assessment of left ventricular systolic dysfunction and left ventricular hypertrophy will be assessed using a mobile phone based 6-lead ECG (KardiaMobile, AliveCor, United States of America) coupled with artificial intelligence (AI) tools developed for ECG analysis48. In the eye, we will assess hypertensive retinopathy using a handheld, mobile phone based, non-mydriatic retinal camera (Remidio, India). Retinal image grading will be conducted by the same staff who graded the reference standard images. Finally, kidney dysfunction (defined as serum creatine levels, eGFR and glomerular function) will be measured using urine dipsticks, and POC clinical chemistry devices (Abbott iStat, Abbott, United States).
Genetic sampling. For the genetic analyses, DNA will be extracted from whole blood samples using Qiagen DNA Blood Mini kit. DNA extraction will make use of blood samples drawn for HMOD measurement and no new blood sample is required. We will then use PCR to determine presence of the genotypes of interest, including malaria and trypanosomiasis protective polymorphisms (Dantu, SCT, APOL1 or APOL2)36,37. These analyses will be conducted in KWTRP.
Health economics assessment. We will use the human capital approach to collect primary data on patient costs including the opportunity cost of time spent administering blood pressure-monitoring methods, missing productive activity and any out-of-pocket costs incurred for undergoing study procedures. Health system cost data will be collected using a micro-costing, ingredient-based approach. Data will be collected from a structured questionnaires administered to three CHWs from each study site, structured observations of CHW and user time spent on BP and HMOD measurement, and a survey using a structured questionnaire to 200 individuals diagnosed with hypertension49.
A detailed statistical analysis plan will be produced before data collection is completed and uploaded to a public repository. A summary of statistical analysis approaches that will be taken for this study is outlined here.
Hypertension prevalence. We will estimate sensitivity; specificity; positive and negative predictive values; and the positive and negative likelihood ratios of each BP measurement approach compared to the reference standard 24-hour BP measurement method.
Identifying hypertension risk factors. The primary outcome will be hypertension, defined as a BP of >140/90mmHG using 24-hour BP measurement, or ongoing therapy with anti-hypertensives. We will determine the relationship between the candidate risk factors and blood pressure and hypertension using multivariate linear and logistic regression methods as appropriate. We will include confounders in the regression models based on results of univariate models and existing literature on the subject.
Prevalence of organ damage. We will analyse the overall prevalence of HMOD and report age- and sex-standardised and stratified prevalence rates. We will also analyse prevalence rates stratified by type of HMOD (left ventricular hypertrophy, impaired left ventricular systolic function, renal dysfunction, hypertensive retinopathy, or arterial stiffness). Finally, we will construct logistic regression models to identify the participant parameters associated with presence of HMOD.
Accuracy of POC diagnostic tools. The diagnostic accuracy of the POC assessments will be estimated using sensitivity; specificity; positive and negative predictive values; and positive and negative likelihood ratios of each POC parameter compared to the conventional measurement. These comparisons will be:
1) Left ventricular systolic function: Smartphone-based ECG (analysed with Mayo Clinic trained AI algorithms) compared to transthoracic echocardiogram (reference standard).
2) Left ventricular hypertrophy: Smartphone-based ECG (analysed with validated and trained AI algorithms) compared to 12-lead ECG and transthoracic echocardiogram (reference standard).
3) Hypertensive retinopathy: Smartphone-based retinal imaging compared to clinical grade retinal camera (reference standard) in detecting HMOD.
4) Renal dysfunction: POC clinical chemistry and urinalysis dipstick compared to conventional clinical laboratory assessment of blood and urine markers for renal function
Cost effectiveness analysis. We will model the comparative cost-effectiveness of different decision-making approaches for initiating treatment of hypertension. We will define cost-effectiveness by comparing the costs (to be obtained from primary data collection) and outcomes (to be obtained from secondary sources) of implementing the four different BP measurement approaches, as well as the reference and PoC assessments of HMOD, to guide initiation of treatment. This analysis will be used in developing the community-centred programme at a later stage of the project.
Approval for research involving humans. Ethical approval for this research has been given by the KEMRI Scientific & Ethics Review Unit (SERU4620; 6 February 2023), the MRCG Ethics Committee and the LSHTM Ethics Committee (28276; 6 March 2023).
Consent. We will obtain written informed consent from all the study participants. CHWs will be trained in the consenting procedures and will be responsible for obtaining consent. We will put in place all the necessary measures to remove potential barriers for informed consent, including language, literacy, and pressure to participate to access care.
All study participants will be provided with comprehensive information about the study in the language they are most comfortable with. This information will explain the study's aims, their specific role in it, and how data being collected will be used. Where a participant is illiterate an independent witness will be present to ensure the correct study information is presented to the participant. This witness will sign the consent form as well.
It will be made clear that participation in any part of the research will be entirely voluntary, will not influence future care and that participants will be free to withdraw at any stage, without giving a reason.
Withdrawals. Participants may withdraw from the study at any time, for any reason. The study team may withdraw participants if they are no longer contactable or if they develop conditions that would exclude them from the study, including a lack of capacity to consent.
Compensation. Participants will not be compensated for home blood pressure measurement visits as they will not disrupt daily activities and will take less than 15 minutes. Participants will be compensated for their time and expenses to attend hospital visits. For each visit, participants will be reimbursed 500 Kenyan shillings or 250 Gambian dalasi plus any travel expenses incurred traveling to and from the research visit.
Urgent care referral. During this research, it is possible that severe medical conditions could be identified. In such cases we will arrange for urgent care and further treatment will follow the locally available standard of care.
This study is part of the wider IHCoR-Africa research group. The outcomes of this specific study will deliver the strongest evidence to date about different diagnostic approaches for hypertension in rural SSA, a unique understanding of the prevalence and characteristics of HMOD in this population, and a robust assessment of the role of innovative, low-cost, POC devices to manage hypertension in rural SSA. In addition, it will provide novel evidence of hypertension phenotypes in rural SSA. The impact of this study (together with other activities of IHCoR-Africa) will improve the long-term health outcomes of people living with hypertension in SSA.
One of the main strengths of IHCoR-Africa is the strong engagement with the community and key stakeholders. The research team has secured support from governmental organizations, civil society, scientific societies, health care providers, and patient groups. Their active engagement in IHCoR-Africa activities before and during the study will be our first step to ensuring early engagement and targeted dissemination of the results. We will present study outputs in scientific meetings of our partners including PASCAR, KCS and AESA and seminars run by the partner institutions. The IHCoR-Africa website, provides up-to-date information on the progress of the study. A formal communication and impact strategy will be developed with the support of the communication departments of participating institutions. Publications produced from IHCoR-Africa will be open-access in accordance with NIHR policy. A publication policy will be agreed by all members of the IHCoR-Africa team prior to the first paper being published.
Ethical approvals are in place for the study. Participant consenting and data collection is underway in both sites, beginning with blood pressure measurements. Screening of HMOD with reference standard and PoC tools will commence in December 2023. Data collection is anticipated to complete by mid-2024, followed by analysis and reporting.
No data are associated with this article. When the results of this study are prepared and published, anonymised data will be made available in line with the policies of the NIHR, LSHTM, KWTRP and MRCG.
Alexander D Perkins, Juliet Otieno Awori and Modou Jobe contributed equally to this work. Pablo Perel and Anthony O Etyang are co-primary investigators of IHCoR-Africa and joint senior authors of this paper. We thank the community members and health workers of Kilifi, Kenya and Kiang West, the Gambia for their time and guidance during consultation efforts. IHCoR-Africa Collaborators: Brahima Diallo, Assan Jaye, Abba Hydara, Bai Cham, Benjamin Tsofa, Edwine Barasa, Samson Kinyanjui, Noni Mumba, Elijah Ogola, Jemima Kamano, Violet Naanyu, Lilian Mbau, Ellen Nolte, Tim Clayton, Melanie Morris, Adrianna Murphy, David Prieto-Merino, Cova Bascaran, Syreen Hassan, Nancy Kagwanja, James Abuje, Mavis Foster-Nyarko, and Saidina Ceesay.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hypertension; Pulmonary hypertension
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Noncommunicable disease research
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hypertension; Pulmonary hypertension
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 17 May 24 |
read | |
|
Version 1 21 Dec 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)