Keywords
Inflammation, Cochlear implants, Hearing, Hearing outcomes, Macrophages, Fibrosis, Transcriptome
Cochlear implantation is a surgical intervention for people with severe-to-profound hearing loss. Electrodes in the cochlea generate electrical currents that stimulate the auditory nerve to elicit hearing. Despite the success of cochlear implants, some people do not receive the expected hearing benefits. One reason for this is that tissues in the cochlea vary in their response to implantation of the electrode array. Many people have a healthy wound-healing response that results in mature scar tissue (fibrosis). However, some individuals have a heightened inflammatory response associated with excessive fibrosis. This leads to greater electrical resistance to the current flow (impedance) and reduces the quality of electrical stimulation, both of which can lead to poorer hearing outcomes with the implant. Excessive inflammation can damage cochlear structures and result in loss of residual hearing.
This study will increase our understanding of why some people have a heightened inflammatory response that leads to poorer hearing. We propose that there are detectable individual inflammatory differences between people when they are implanted, which may result in variable hearing outcomes following implantation. If we could understand and identify these differences, we could detect people who may be at risk of less favorable outcomes and use therapies to modulate inflammation and improve outcomes.
A cross-sectional study of children and young people undergoing cochlear implantation. On the day of surgery, a middle ear mucosa sample, swabs of the nasopharynx and middle ear, cochlear fluid, and blood samples will be collected.
Samples will be analyzed using molecular techniques to determine the inflammatory status of the person at the time of implantation. Clinical hearing data will be collected for up to five years after implantation to explore the relationship between inflammation at the time of implantation and long-term hearing outcomes.
Despite the success of cochlear implants, some patients do not achieve the expected hearing benefits. This is detrimental to a person’s well-being and makes it challenging for them to interact daily in the ‘hearing’ world. Poor outcomes with implants are costly to the individual, society, and health system. The factors that contribute to underperformance are not well understood.
One factor could be how the body responds to the insertion of the implant into the cochlea. Most people have a healthy tissue response, which results in scar tissue (fibrosis) around the implant. In some individuals, the response is more inflammatory, leading to increased fibrosis in the cochlea. This causes damage to the surrounding tissue and can lead to further hearing loss and poor hearing with the implant.
A better understanding of the factors that cause poorer hearing may enable the use of anti-inflammatory drugs before and after surgery to give people better hearing with their implants.
CHIEF (cochlear implants and inner ear inflammation) is a cross-sectional study of children and young people undergoing cochlear implantation. On the day of surgery, tissue and fluid samples will be collected, including the middle ear mucosa, middle ear, nasal swab, cochlear fluid, and blood sample. In cases where an implant is removed because of implant failure, the implant and tissue attached will be collected. Following implantation, we will collect clinical outcome measures for up to five years.
We will test our hypothesis that the insertion of an electrode array into the cochlea causes an inflammatory tissue response that varies due to individual inflammatory differences at the time of implantation. We aim to characterize the inflammatory state of the ear. We will combine biological data with health and hearing data to investigate the relationship between the inflammatory state and long-term hearing outcomes.
Inflammation, Cochlear implants, Hearing, Hearing outcomes, Macrophages, Fibrosis, Transcriptome
In the revised version and response to reviewers we have:
- Added text to clarify and define the specific variables depicted by hearing outcomes and added a section titled ‘clinical data collection’ to provide further detail about what routinely collected clinical data, and at what time-points, we will be collecting to measure hearing outcomes in the study participants.
- Added a section titled ‘Standard protocol for cochlear implantation at Manchester University Hospital’ to clarify the standard surgical protocols at this site.
- Added text to the section with the sub-heading ‘Secondary outcome’ to explain how the characterisation of the immune state of each study participant will inform the analytical plan to achieve the secondary outcome, which is identify the relationship between the immune state of the ear in children and young people at the time of implantation and hearing outcomes following implantation.
- Clarified how the range of biographic, etiologic, device related and surgical factors will be considered when examining the role of immune state at implantation influencing hearing outcomes in this observational, pilot study.
- Added in text in the patient and public involvement section to describe the new ALL_EARS@UoS Young People’s Advisory Group (YPAG) which we have launched for people aged 14 – 16 years old with lived experience of deafness. This group will directly support the CHIEF study.
See the authors' detailed response to the review by Teresa Ching
Cochlear implantation is a treatment developed for people with profound deafness. Cochlear implants are effective in helping young children to learn to talk and listen, older children to achieve at school, and children and young people of all ages to socialize. Children who hear well with implants, are likely to meet key developmental milestones in line with their unaided hearing peers1. This contributes to the transition to adults who are better able to succeed in society.
Cochlear implantation involves the surgical implantation of a wire (electrode array) into the hearing organ (cochlea) in the deep part of the ear (inner ear). The electrode array carries a signal from an electronics package implanted under the skin (receiver-stimulator package), which itself receives a coded signal from a bespoke hearing aid worn on the ear (audio processor). Electrical signals stimulate hearing pathways in the brain, resulting in sound perception.
Some people do not do as well as expected following surgery or the hearing benefit from the implant tail-off over time2–4. This can be detrimental to the well-being of the person, resulting in non-use of the cochlear implant or even the need to undergo another operation to insert a new cochlear implant5. This can mean the potential benefits of restored hearing are lost.
How the cochlear implant electrode array interacts with the delicate tissues in the inner ear is crucial to the effectiveness of the electrical stimulation of the hearing pathway to the brain and the preservation of any remaining natural hearing. The immune system and inflammatory response within the inner ear are likely key factors in the interaction between the electrode array and fine structures of the ear6–9. We know that implantation causes an inflammatory response, but most of our understanding of this comes from studies of implantation in animals10,11 and cadaveric temporal bone studies12,13. Importantly, these studies have not enabled us to understand how the inflammatory response might vary between individuals, each of whom has their own immune history14. We need to understand and ideally be able to predict or anticipate the individual inflammatory response, as this could lead to improved clinical management and better hearing outcomes for more people who use cochlear implants.
Our hypothesis is that the insertion of an electrode array causes an inflammatory response that varies due to individual differences between people at the time of implantation.
There is an urgent need to investigate the effect of the immune state of the inner ear on hearing outcomes following cochlear implantation. To achieve this, we need to study the inflammatory state of the ear in children and young people undergoing implantation. Importantly, we need to see how this varies between children and young people, and how it is associated with hearing outcomes in people with a history of middle ear inflammation (acute otitis media and otitis media with effusion)15,16. Hearing outcomes is the combination of audiological tests, speech perception tests, language development tests, datalogging and parental assessments that allow cochlear implant professionals to measure and determine how much benefit someone is getting from their cochlear implant. Currently, there are no studies that have investigated hearing outcomes over time in a cohort of pediatric cochlear implant patients with and without a history of middle ear inflammation. The CHIEF study will allow us to investigate this.
The desired outcome from this study would be to understand when targeted treatment (e.g., steroids) before, during, or after surgery is needed to ensure the best outcome for the person with their implant.
ALL_EARS@UoS is a patient and public involvement and engagement (PPIE) group that was first established at the University of Southampton in March 2022. The group is committed to improving the understanding of the mechanisms, lived experience, and management of hearing loss by contributing to and influencing hearing loss research17. The chief investigator for this project (TN) and the postdoctoral fellow (KH), who will work on the project, have been central to ALL_EARS@UoS. The CHIEF study was presented and discussed with the group for feedback at our regular PPIE meetings. All documents for the study were shared with members of the group for detailed feedback. One group member attended an ethical approval panel meeting with the chief investigator of the project. In March 2025, we launched ALL_EARS@UoS Young People’s Advisory Group (YGAG) which is PPIE group for young people aged between 14 – 16 years old (https://generic.wordpress.soton.ac.uk/all-ears/2025/03/07/join-our-new-young-peoples-advisory-group-ypag/). Members of ALL_EARS@UoS YPAG will support this study alongside our wider research.
The study will be conducted at the Manchester University Hospital NHS Foundation Trust (MFT) and the University of Southampton. The Manchester pediatric cochlear implant program was established in 1991, and it serves a diverse population and implants in over 60 children per year. It has a sustained record in undertaking research to improve outcomes for children and young people with implants. The University of Southampton has significant expertise in inflammatory biology, microbiology, and proteomics. It is a core partner of the National Biofilm Innovation Center and home to one of the 19 auditory implant services in the UK (University of Southampton Auditory Implant Service).
Participant recruitment, sample collection, and clinical management of the participants will be carried out in the MFT. Sample analysis and initial data interpretation will be carried out in Southampton with data sharing between centers. Only fully anonymized patient and sample data will be shared with Southampton, and all identifiable information will be limited to the clinical care team in the MFT.
Observational, cross-sectional study of children and young people undergoing cochlear implantation. Children and young people who meet the inclusion criteria and who have consented and/or give assent will be recruited to the study. The surgical protocol and the participants’ routine clinical care will not be altered by being study participants. Figure 1 outlines the participant pathway from determining eligibility and recruitment into the study through sample collection on the day of surgery, followed by the collection of clinical and health data post-implantation.
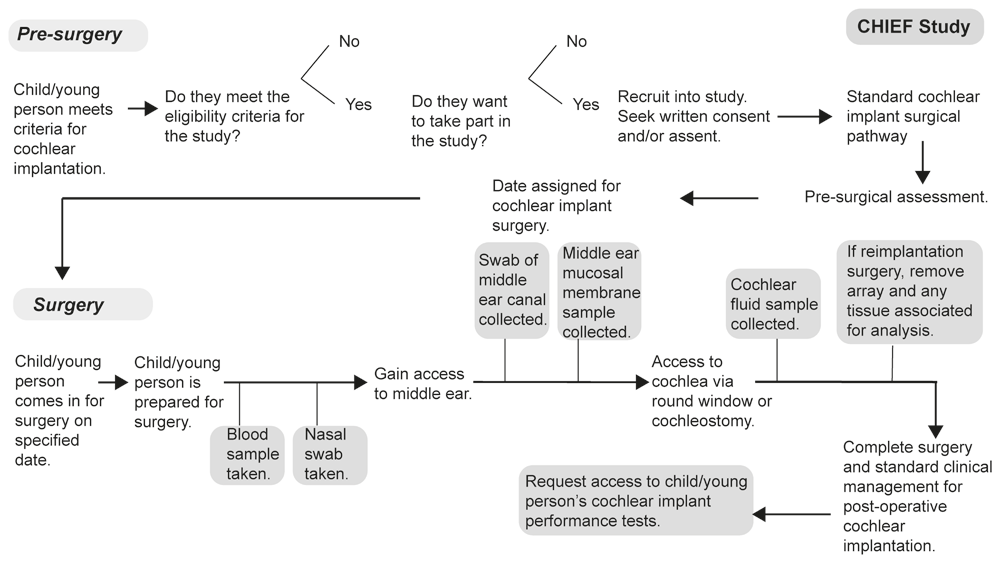
This protocol was written according to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines. The STROBE checklist was filled out where applicable to this protocol (https://doi.org/10.5258/SOTON/D3345).
Eligibility criteria
Children and young people undergoing cochlear implantation under the care of the Manchester University NHS Foundation Trust (MFT) will be screened to determine if they are eligible to participate in the study. See Table 1 for inclusion and exclusion criteria.
If the patient meets the eligibility criteria mentioned above, the clinical care team will provide the necessary information about the study to the children and young people and/or their parents or guardians.
Recruitment
We aim to recruit from children and young people who are eligible within the 24-month period of sample collection. We estimate 75 participants over two years of recruitment to the study. This is based on the recent rates of pediatric cochlear implantation at the MFT. The results of this observational study will provide the sample size and recruitment criteria for future interventional studies.
Recruitment will be done by advertising the study to young people, parents, and guardians of children who meet the eligibility criteria. The clinical care team responsible for the care of patients will determine eligibility. The study details will be shared during the clinic visit with children and young people who have been identified by the clinic coordinator as meeting the criteria.
All eligible patients and/or their parents or guardians will receive information regarding the study, including age-appropriate participant information sheets, consent, and assent forms. Information about the study will be publicized on posters in the clinic, with links through quick response [QR] codes to more information about the study. The information will be hosted on our publicly available PPIE group, ALL_EARS@UoS, website [https://generic.wordpress.soton.ac.uk/all-ears/2024/11/12/chief-study-cochlear-implants-and-inner-ear-inflammation/]. The study documents were developed with the input and scrutiny of members of ALL_EARS@UoS.
Sample collection
A sample of the middle ear mucous membranes, swabs of the nasopharynx and middle ear canal, cochlear fluid, and a blood sample will be collected at the time of surgery for cochlear implantation. Samples from the ear will only be collected from the ear or ears being implanted.
In cases where an implant is removed because of implant failure, along with the samples listed above, we will collect any tissue attached to the implant being removed.
Sample management and storage
Samples will be collected and either transferred for routine analysis (blood sample-hematology) or post-collection processing (tissue samples and any explanted arrays) and then stored using the biobanking facility (4°C or -80°C) at MFT.
The nasal and middle ear swab, middle ear mucosa, and tissue from the explanted array will be stored before being transferred to the University of Southampton for analysis. The cochlear fluid (CF) will be stored for up to one year at MFT and transferred to Southampton at the end of year one and year two.
Sample processing
The middle ear mucous membrane and tissue associated with the implant in cases of re-implantation will be collected into pre-prepared collection tubes of 10% neutral buffered formalin18 during surgery for tissue fixation (overnight (12–16hrs at 20–25°C), before being transferred to 70% ethanol for storage (4°C) until processing. The samples will be processed into paraffin wax tissue and prepared as microarrays for histology and spatial transcriptomics.
Samples collected through swabs of the middle ear and nasopharynx will be stored before bacterial gene and viral analyses. The samples will be processed to isolate DNA using commercial kits for low (low blood contamination) and high biomass (fluid samples and where blood is present), and the 16S DNA quantity will be determined.
Cochlear fluid collected immediately prior to insertion of the cochlear implant array will be analyzed for the presence of proteins. The samples will be processed using an existing technique19 and the data will be analyzed using bioinformatics.
Immune cell identification and characterisation from tissue analysis
Histological analysis - Using our published method20, antibodies for macrophages, activated macrophages, T cells, fibroblasts, endothelial cells, and a marker of cellular proliferation and appropriate counterstain will be used to identify the gross tissue morphology and distribution of cells within <4μm tissue sections. Appropriate controls will be used, and the samples will be processed in batches to reduce inter-sample variation. Cell counts will be performed on each sample, with the final data expressed as cells/unit area of tissue after image capture and analysis of the tissue sections using quantitative image analysis microscopy with a custom ImageJ plugin.
Spatial transcriptomic analysis – Gene expression and spatial transcriptome profiles at single-cell resolution of immune cells (macrophages) from middle ear mucosa tissue samples will be generated in a subset of samples21–23. We will analyze the expression profiles to provide unbiased characterization of the activation state and ‘memory’ of macrophages between, and within, samples using the high-plex, spatial molecular imaging platform, CosMx (manufactured by Bruker Spatial Biology)24.
Initial analysis and data visualization will be performed using AtoMx, a cloud-based, fully integrated spatial informatics platform. We will work through a data analysis pipeline, using TileDB as the primary data structure. The data will go through the quality control module to flag any unreliable negative probes, cells, fields of view, and target genes. The data will be normalized before Principal Component Analysis (PCA). This will be used as an input to uniform manifold approximation and projection (UMAP) to enable the visualization of clusters of related cells within the tissue. The InSituType Cell Typing module will be used for cell typing. Differential gene expression testing will be used to determine the genes that were expressed at different levels between and within the samples.
The primary aim of this study is to understand whether inflammation is a factor in inter-individual variability in response to cochlear implantation. Macrophages are long-lived cells and have ‘a memory’ of exposure to injury or infection. This ‘memory,’ known as activated or primed6,25,26, can cause the macrophage to generate a larger inflammatory response if it is stimulated by a second injury or infection. The primary inflammatory response in macrophages is essential to drive repair, remodelling, and recovery, as might occur when macrophages respond to clear infections or pathogens in the inner ear. However, an excessive inflammatory response in primed macrophages causes bystander damage to the delicate tissues of the cochlea27,28 and may contribute to the development of scar tissue or fibrosis around the implant. Fibrosis around the array insulates the electrodes and alters the release of electrical current from the electrodes to their intended target for stimulation, the spiral ganglion cells of the auditory nerve. This change in electrical behavior can be measured as an increase in impedance29–31. Evidence suggests that fibrosis is associated with electrode migration32–34. Altered current spread and movement in the electrode array are likely to be perceived and measured as a change or poorer hearing outcome with the implant. The gene expression patterns, determined through bioinformatic analyses, of the macrophages will enable us to achieve our primary outcome for this part of the study, which is to characterize macrophage ‘memory’, and how this differs within and between cases. This phase of the work is a pilot study; if successful, we aim to secure additional funding to characterize the response in all the study cases.
A secondary outcome of this phase of the study will be a dataset that captures the gene expression patterns of other cell types in the tissue. This dataset will be used in the development of follow-up studies from this study. The data will be made accessible to other researchers with appropriate ethical approval on request via data curation through the University of Southampton library. We will monitor and consider the most appropriate data-hosting site as the project evolves. If a bespoke discipline-specific externally hosted data repository becomes available, we can store fuller datasets externally. This will be incorporated in future funding applications.
Identification of bacterial species
Nasal and middle ear swabs will be analyzed using culture-independent 16S ribosomal RNA (rRNA) gene amplicon sequencing to identify the bacterial species within these sites. This method enables the identification of different strains of bacteria on the mucosal surface (swabs) or within middle ear fluid35. The samples will be processed to isolate DNA using commercial kits for low (low blood contamination) and high biomass (fluid samples and where blood is present), and the 16S DNA quantity will be determined. Quantitative PCR will be carried out to determine the bacterial populations and relative proportions of the populations. A known issue with using a non-culture method for the identification of bacterial strains and species is an increased likelihood of false-positive results36. Anonymized positive control samples and sample spiking will be included within our analysis protocols to mitigate the risk of false positives.
Identification of viruses
Samples collected from swabs of the middle ear and nasopharynx will be stored before viral analysis.
A key aim is to understand how bacterial or viral populations relate to the state of inflammation of the tissue in the middle ear in children and young people at the time of cochlear implantation. Bacteria in the middle ear have been studied in children37 and there is some published work on bacterial analysis in adults undergoing implantation35. However, very few children and young people undergoing implantation have been included in this work and the relationship between inflammation and hearing outcomes is not known and has not been systematically studied. This study provides the first data of this type.
The secondary aim is to investigate the relationship between the microbiota of the middle ear and nasopharynx. A secondary outcome of this study is to identify whether there is a distinct middle ear microbiota or biomarker associated with a poorer hearing outcome with a cochlear implant. This would be a significant new finding that may influence the clinical management of the middle ear prior to surgery.
If the middle ear microbiota are identified as a biomarker for hearing outcomes with a cochlear implant, there would be further limitations and challenges to address, as sampling the middle ear is an invasive procedure. Therefore, if during this study, we find that the nasopharynx microbiota mirrors that of the middle ear it may be that routine nasal swabs could be collected at home from children with a history of middle ear infection and sent to a laboratory for analysis. This could provide a more tolerable, easier to complete pre-surgical test, and one that can be readily repeated to monitor improvements after, for example, treatment with antibiotics.
Identification of inflammatory markers in the blood
Blood samples will be collected on the day of surgery and analyzed in the hospital hematology department to provide a full blood count on the day of surgery. The counts will be analyzed, along with the differential white blood cell count and neutrophil-to-leukocyte ratio, to determine the presence of systemic inflammation at the time of surgery. Previous studies, including cross-sectional analyses, have investigated the relationship between inflammation and hearing loss38–41. The mechanism linking inflammation to changes in the cochlea, which results in poorer sensorineural hearing loss later in life, is not understood. This study will add new information about these changes in early life.
A key aim of this study is to determine whether there is an association between the levels of systemic inflammation at the time of implantation and hearing outcome following implantation. Does increased levels of systemic inflammation correlate with an increased immune/inflammatory state in the middle and inner ear and does this influence the tissue response following cochlear implantation?
Identification of inflammatory markers in the cochlea
Proteomics will be used to characterize the levels of inflammatory markers in the cochlea19,42–44. Proteomics is an unbiased technique that enables the detection, identification, and quantification of proteins within a sample. The high sensitivity of this technique enables the detection and quantification of very low levels of protein. This unbiased approach to protein detection means that all proteins in the sample above the detection limits can be identified, and their relative expression determined. A limitation to this can be introduced by samples that require large proteins, such as those found in blood, to be stripped prior to analysis. Low expression of proteins, or proteins with a high affinity for blood proteins, may result in the loss or reduction of some proteins. We will use existing techniques to collect and analyze samples19 with specialist technical support from our proteomics and bioinformatics research units. These results will enable us to determine whether the cochlea shows evidence of inflammation, as determined by the proteins present in the cochlear fluid45 prior to the surgical insertion of the implant.
A key aim of this study is to understand whether some of the differences in response to cochlear implantation are due to individual differences in inflammation in the cochlea at the time of implantation. There are no published or established techniques to measure or detect these differences in the intact cochlea prior to implantation. However, there is evidence that there may be differences between people, as in some cases, people who have had meningitis, labyrinthitis, and other auto-immune-induced SNHL have fibrosis, or the growth of tissue within the cochlea46 at the time of cochlear implant insertion. Fibrosis occurs in many organs, such as the liver and lungs47 and inflammation is a consistent feature irrespective of which organ is affected. On this basis, we predict that some people will be more inflamed, and that there is a need for a method to anticipate this. We will integrate the information from the cochlea at the time of implantation with the data collected from tissue and swab samples. Together, this may enable us to develop a method of predicting children and young people who have a greater likelihood of inflammation in their cochlea prior to implantation or a stronger or more prolonged inflammatory response after implantation, both of which may mean they hear less well with their implants and/or initially preserved natural low-frequency hearing is not maintained48. In the longer term, it may be possible to identify these children and young people for more aggressive anti-inflammatory management when they have implants.
A secondary outcome of this phase of the study will be a dataset that captures the protein expression of the fluid in the cochlea in a cohort of children and young people. This dataset will be developed and used in the development of follow-up studies from this work, and the data will be made accessible to other researchers on request via data curation through the University of Southampton library.
Standard protocols for cochlear implantation at Manchester University Hospital
Surgeons routinely use soft surgery technique for all patients, even for those who do not have residual hearing. This is so that they preserve neural tissue in all patients. All children get 1 dose of peroperative co-amoxiclav 30mg/kg up to a maximum of 1.2g or an alternative if penicillin allergic. In addition, the patients will also get a peroperative dose of steroids – 0.15mg/kg, up to a maximum of 6.6mg.
Post-operatively, all children are discharged on 3 doses of oral dexamethasone, 0.15mg.kg and 3 doses of antibiotic. If there are signs of acute inflammation during surgery in the form of inflamed mucosa and sero-mucinous otitis media, the surgeons will give 1 week’s treatment with oral antibiotics post-operatively.
Clinical data collection
To measure hearing outcomes, a battery of age-appropriate audiological, speech perception and language development tests are used, in addition to datalogging measurements. To explore the relationship between the immune state of the middle ear tissue and long-term hearing outcomes, we will request access to anonymized, routine clinical outcome measures for up to five years post-implantation of study participants. This will include audiological and device data; aided levels from speech and language tests (ASSE and AB words); datalogging (device usage e.g. average daily use) and device measurements including electrode impedances and deactivations (number of electrodes and position). Together with a log of any recorded post-implantation complications.
All data collected post-implantation will be from routinely collected clinical data. The device measurements will be collected when a patient comes in for an appointment or via remote appointments. The time points of these measurements from the day of implantation may vary but will loosely follow the appointment structure which is: switch on 7 – 14 days post-implantation, 1 month, 3 months, 6 months, 2 years, 4 years, 6 years (new processor).
We will access data from medical records including date of birth, ethnicity, history and cause/etiology of deafness, history of ear disease (infections, surgery), and history of other infections (meningitis, cytomegalovirus, measles). Descriptive statistics will be used to describe hearing performance and device measurements.
Research questions:
1. What is the immune state of the middle ear in children and young people undergoing cochlear implantation?
2. How distinct or similar are the microbiota (bacteria and viruses) of the nasopharynx and middle ear within individuals?
3. What is the relationship between microbiota and the immune status of the middle ear tissue?
4. What is the inflammatory state of the cochlear fluid at time of surgery?
5. What is the inflammatory state of the tissue associated with the cochlear implant electrode array? How does this relate to the other sites tested in this study?
6. What is the relationship between the inflammatory state of the middle ear and the outcome of cochlear implantation?
Objectives:
Use molecular techniques to;
1. identify the immune cells that are present.
2. characterize the inflammatory state of these cells in the samples of the middle ear mucous membrane (MM) or tissue attached to an implant (FT).
3. identify bacteria that are present and the size/distribution of the populations in the middle ear (ME) and nasal swab (NS).
4. identify viruses that are present and the size/distribution of the populations in the middle ear (ME) and nasal swab (NS).
5. detect the presence and levels of inflammatory markers in cochlear fluid (CF) and blood samples (S).
Analyse clinical outcomes routinely measured across five years for the study participants.
Primary outcome
This study aims to identify differences in ear inflammation between children and young people at the time of cochlear implantation.
Secondary outcome
A secondary outcome of this study is to identify the relationship between the inflammatory status and microbiota of the ear.
The secondary outcome of this study is to identify the relationship between the immune state of the ear in children and young people at the time of implantation and hearing outcomes following implantation.
The characterisation of the immune state of each study participant will inform the analytical plan to achieve this secondary outcome. Due to anticipated variability in immune state it may be that this is the pilot study to informs a larger powered study. This step is necessary as there is no prior equivalent data. Likewise the analysis of the hearing outcome will depend on the age of participants and the battery of hearing, speech and language assessments over time.
Limitations of the study design
This study is an observational study. The results of this study will inform the sample size and recruitment criteria of future interventional studies. In addition, the complete analysis and interpretation of the samples in this study will require several highly specialized and expensive techniques. This will require follow-on funding bids informed by data from this study.
Improve patient outcomes
This study will allow us to combine spatial gene and protein expression data with clinical and hearing data to address the long-term aim of improving patient outcomes by identifying if there is a predictable relationship between inflammatory status and hearing outcomes following implantation. This is the first study that aims to characterize the immune state of the ear at the time of implantation and to correlate it with hearing outcomes with a cochlear implant.
Initially, we will determine whether the inflammatory state of the middle ear at the time of implantation is different between children and young people using histology and spatial transcriptomics. We will combine molecular data with clinical and hearing data to determine whether there is an association between hearing and surgical outcomes following implantation. This provides essential pilot data to support further funding applications.
We hypothesize that some children and young people who have a heightened inflammatory tissue environment in the ear due to previous inflammatory insults such as recurrent infections may elicit an increased inflammatory tissue response to cochlear implantation6, resulting in increased fibrosis. This could result in poorer hearing outcomes with the implant, both in terms of initial preservation and subsequent maintenance of residual natural hearing after implantation48,49 and worsening of the quality of electrical stimulation over time50,51. If we can identify patients who are at greater risk of an increased inflammatory tissue response to implantation and therefore poor hearing outcomes, we could intervene prior to, or at, surgery with existing and novel anti-inflammatory and/or antimicrobial therapies and ensure closer post-implantation monitoring to improve hearing outcomes following implantation.
Build a rich database containing clinical and medical records of children and young people who have undergone cochlear implantation, alongside building a rich tissue bank
This longitudinal, observational study design will allow us to collect and bank multiple tissue and fluid samples alongside detailed participant clinical, hearing, and medical data for five years. Using this data, we will produce a rich database that will allow us to ask hypothesis-driven research questions. We will use pilot data obtained from initial histological and transcriptomic tissue analyses to apply for larger funding bids.
Contribute novel data sets
Our study design, alongside the use of spatial transcriptomics, will contribute novel data sets, including the first spatial transcriptome profile of macrophages in the middle ear tissue of deaf children undergoing cochlear implantation, as well as the transcriptome profile of other cell types, including fibroblasts, which are relevant for cancer and respiratory biology.
Full ethical approval was obtained from the Integrated Research Application System (IRAS) [330110] and local ethical approval was obtained from University of Southampton via Ethics and Research Governance Online (ERGO) [89599]. Approval was obtained on 20.09.2024.
Written consent will be obtained from the parents or guardians of the children and young people (aged 1–15 years old) that will be recruited into this study. In addition, written assent will be obtained from children aged 5–15 years old.
Written consent will be obtained from children aged 16 years old.
A preprint of this article is available on MedXRiv52. The results will be submitted to international peer-reviewed journals and presented at conferences. The results will be presented to a lay audience via the patient and public involvement and engagement group (ALL_EARS@UoS) website (https://generic.wordpress.soton.ac.uk/all-ears/).
In addition to this, we will share findings and progress of the study through the various public engagement activities that we take part in each year alongside ALL_EARS@UoS members.
No data are associated with this article. Future datasets from the CHIEF study will be published in the University of Southampton PURE repository.
University of Southampton Institutional Repository: STROBE checklist for ‘Protocol for CHIEF (cochlear implants and inner ear inflammation) study;an observational, cross-sectional study of children and young people undergoing cochlear implantation. https://doi.org/10.5258/SOTON/D3345
This project contains the following underlying data:
CHIEF_Protocol_-_STROBE-checklist.docx - Other
STROBE_Checklist_Readme.txt – Dataset
Data are available under the terms of the Creative Commons Zero "No rights reserved data waiver (CC0 1.0 Public domain dedication).
TAN, IB, KH and JN conceived the study. TAN and IB previously carried out a small pilot study that confirmed the feasibility of cochlear fluid sampling during routine surgery for cochlear implantation. IB and JN considered the timeline, feasibility, and method of sample collection during the surgical procedure. TAN and KH demonstrated the feasibility of histological analysis and identified inflammation associated with the tissue on an explanted device. CF contributed to the experimental design, including the capture and management of the data. TAN generated the first draft of the protocol and all authors contributed to this draft.
The authors are grateful to members of the ALL_EARS@UoS PPIE group who shared their experiences of hearing loss and cochlear implantation, contributed to the discussion, and provided feedback about the CHIEF study. The authors are grateful to the group member SW, who attended the ethical approval panel meeting. The authors are grateful to the Pediatric and Adolescent Cochlear Implant Team of Manchester for supporting this study. We thank the cochlear implant patients who will take part in the study.
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
References
1. Zhang D, Chen D, Wang K, Pan J, et al.: Electrical stimulation of cochlear implant promotes activation of macrophages and fibroblasts under inflammation.Laryngoscope Investig Otolaryngol. 2023; 8 (5): 1390-1400 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: hearing disorders
Is the rationale for, and objectives of, the study clearly described?
Partly
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cochlear implants, pediatric outcomes, adult outcomes, aural rehabilitation for children who are deaf or hard of hearing
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 15 May 25 |
||
|
Version 1 04 Mar 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)