Keywords
Vaccine impact, Vulnerability index, Vaccine coverage, Vulnerable communities, Uganda, Kenya
Despite global efforts to improve on vaccine impact, many African countries have failed to achieve equitable vaccine benefits. Reduced vaccine impact may result from interplay between structural, social, and biological factors, that limit communities from fully benefiting from vaccination programs. However, the combined influence of these factors to reduced vaccine impact and the spatial distribution of vulnerable communities remains poorly understood. We developed a Community Vaccine Impact Vulnerability Index (CVIVI) that integrates data on multiple risk factors associated with reduced vaccine impact, to identify communities at risk, and key drivers of vulnerability.
The index was constructed using 17 indicators selected through literature review and categorised into structural, social, and biological domains. Secondary data was obtained from national Demographic and Health surveys from Uganda (2016) and Kenya (2022), covering 123 districts and 47 counties, respectively. Percentile rank methodology was used to construct domain-specific and overall vulnerability indices.. Geo-spatial techniques were used to classify and map districts/counties from least to most vulnerable.
We observed distinct geographical patterns in vulnerability.. In Kenya, the most vulnerable counties were clustered in the northeast and eastern counties such as Turkana, Mandera, and West Polot. In Uganda, vulnerability was more dispersed, with the most vulnerable districts in the northeast (e.g. Amudat, Lamwo) and southwest e.g. Buliisa,Kyenjojo). Key drivers of vulnerability included long distance to health facilities, low maternal education, poverty, malnutrition, limited access to postnatal care, and limited access to mass media. Some areas with high vaccine coverage also showed high vulnerability, suggesting coverage data may not reliably reflect vaccine impact. Each community showed a unique vulnerability profile, shaped by different combinations of social, structural and biological factors, highlighting the need for context specific interventions.
The CVIVI is a useful tool for identifying vulnerable communities and underlying factors. It can guide the design of tailored strategies to improve vaccine impact in vulnerable settings.
Vaccination saves millions of lives every year; however, in many African countries, people are still dying from vaccine-preventable diseases. This is often due to low vaccine coverage and differences in how well individuals respond to vaccines. Several factors may contribute to these challenges, including poor access to healthcare services, high levels of poverty, and malnutrition, which collectively are likely to reduce benefits from vaccination programs. Identifying which communities at a risk of reduced vaccine impact and the main driver for their vulnerability requires data-driven approaches that integrate data on multiple risk factors into a single value. In this study, we developed the Community Vaccine Impact Vulnerability Index (CVIVI), which helps to identify geographical areas where communities are less likely to benefit fully from vaccines and vaccination programs. Using the CVIVI, we found out that factors such as high levels of poverty, low maternal education, limited access to mass media, and malnutrition often intersect in specific districts and counties, making these areas susceptible to reduced vaccine impact. For instance, counties such as Turkana in the northwest and Tana River in southeastern Kenya, along with Buliisa district in western Uganda and Amudat district in northern Uganda, were identified as most vulnerable. The index thus enables policymakers and researchers to identify communities at a risk of reduced vaccine impact and highlight barriers contributing to this vulnerability. With this information, the index would serve as a starting point for policymakers, implementers, researchers, and other stakeholders to understand vulnerability to vaccine impact, and design better tailored interventions, ensuring that every community fully benefits from vaccination programs
Vaccine impact, Vulnerability index, Vaccine coverage, Vulnerable communities, Uganda, Kenya
We revised the manuscript in response to the reviewer’s comments. In the methodology section, we expanded the rationale for indicator selection and explained how their relevance was determined beyond the literature review. We also justified the use of equal weighting in the index construction and discussed whether alternative approaches, such as PCA, were considered. Additionally, we clarified the rationale for using secondary data and addressed limitations related to data consistency, particularly in vaccine coverage estimates. A summary of the spatial data handling processes was also included. In the conclusion section, we linked the recommended actions to the broader public health goals outlined in the WHO Immunisation Agenda 2030. Finally, we added funding information.
See the authors' detailed response to the review by Morufu Olalekan Raimi
Vaccination is one of the most effective public health interventions, significantly reducing mortality and morbidity from vaccine preventable diseases (VPDs)1,2. Between 2000 and 2019, vaccination efforts in 98 low and middle-income countries (LMICs) averted about 37 million deaths, with substantial reductions among children under 5 years3. Despite significant progress and efforts in expanding global immunisation coverage, disparities in access to and impact of vaccination persist4,5. These disparities are especially pronounced in Low-middle income countries (LMICs), where millions of children still miss out on essential vaccines (ref). For instance, in 2023, approximately 6.7 million African children were classified as zero dose i.e. had not received any single dose of vaccine, partly die to the disruptions from the COVID19 pandemic6. Vaccine coverage also varies significantly both between and within African countries, reflecting underlying social and structural inequalities. For example, In Uganda, only 63% of the children aged 12-23 months received all the essential vaccines in 2022, with district level vaccine coverage ranging from 33% to 84%7. Similarly, in Kenya, full immunisation coverage was 80% in 2022, but country level vaccine coverage varied from 29% to 94.9%7. These geographic inequalities in vaccine coverage are influenced by social and structural determinants such as maternal education, income level and health system accessibility8,9. Moreover, vaccination disparities are not limited to vaccine access and uptake alone but also extend to reduced vaccine efficacy and immune responses, resulting into reduced full benefits of immunisation programs. Existing evidence shows that some vaccines may have reduced efficacy and immune responses in African populations compared to high income settings. For example, Bacillus Calmette-Guerin (BCG) vaccine provided almost 100% protection against tuberculosis among UK school children but achieved only 50% efficacy among Malawi adolescents10. Similar patterns are observed with other vaccines such as rotavirus, polio, and Hepatitis B11,12. This variation may be due to biological factors such as age, malnutrition, exposure to infections, which impair immune responses in certain populations and settings14–16. Thus, social, structural, and biological vulnerabilities likely interrelate in various ways to reduce the overall benefits from vaccination programs. Recognizing these challenges, the WHO's Immunization Agenda 2030 (IA2030) and Gavi’s 5.0 strategy call for equity-focused approaches that go beyond national averages to identify and prioritize vulnerable communities16,17. However, few tools exist to systematically map community-level vulnerability to reduced vaccine impact, particularly through a multidimensional approach that integrates social, structural, and biological vulnerabilities. Geo-spatial health analysis has emerged as a pivotal approach for identifying and visualizing spatial inequalities in health service access, immunisation coverage, and disease burden especially in LMICs5,18,19. In public health, researchershave developed vulnerability indices to map and prioritize populations at risk of poor health outcomes and guide resource allocation. For instance, the Social Vulnerability Index (SVI) developed by the US Centre for Disease Control and Prevention (CDC) was developed to help identify and map communities that need support during emergencies or disease outbreaks20. In addition, the maternal vulnerability index has been developed to highlight areas with poor maternal and child health outcomes in United States21. More recently, in the vaccination space, the zero dose vulnerability index was developed to identify zero dose and missed communities in LMICs22. While this index provides critical insights on vaccine coverage inequalities, it may not capture areas where vaccines are received but offer weak protection due to complex interplay between social, structural and biological vulnerabilities. The NIHR Global Health Research Group on Vaccines for Vulnerable people in Africa (VAnguard)23 study was designed to address this gap by identifying modifiable social, structural and biological determinants of impaired vaccine impact in vulnerable communities and design strategies to address them. To inform the VAnguard study, the Community Vaccine impact Vulnerability Index (CVIVI) was developed. The CVIVI integrates data on structural, social, and biological factors to identify communities at a risk of reduced vaccine benefits and the key underlying factors contributing to these vulnerabilities. In this work, we define vulnerability as increased likelihood of communities or individuals to experience reduced benefits from vaccination programs due to the interacting social, structural and biological factors. By understanding the interplay of the underlying factors that contribute vulnerability to reduced vaccine impact, the CVIVI provides insights into specific challenges faced by communities. The information can be utilized to identify geographical areas at a greater risk of experience suboptimal vaccine impact and design tailored strategies to address them.
This study involves a secondary analysis of existing data. Patients and/or Public were not involved in research design, conduct, recruitment and dissemination plans.
The Community Vaccine Impact Vulnerability Index (CVIVI) was developed and implemented following a structured process (Figure 1), comprising six key aspects: (1) indicator selection, (2) data collection, (3) descriptive correlation analysis, (4) index construction, (5) spatial analysis, and (6) identification of vulnerable communities in Uganda and Kenya.
Indicators were selected based on three criteria (i) evidence from literature; (ii) availability and data quality of district/county level data, and (iii) relevance to the local context in Uganda and Kenya. Literature review identified factors known to directly or indirectly influence vaccine impact, either through vaccine coverage or immune responses. In addition, we also assessed the prevalence and variability of each indicator across communities to ensure they could meaningfully distinguish levels of vulnerability. Given the limited availability of quality district/county level data, we prioritised indicators for which reliable data existed. Based on these criteria, we selected 16 indicators, categorised into three domains: structural (seven indicators), social (six indicators), and biological (three indicators). These indicators reflect multidimensional factors that have each been demonstrated to influence vaccine impact. Table 1 shows the vulnerability domains, indicators used, and information on the data sources.
| Domain | Indicators | Definition | Source |
|---|---|---|---|
| Biological | Stunting | Percentage of children under five years who are stunted (greater than 2SD below the median height for age). | KDHS, 20227, UDHS, 201628 |
| Anemia | Percentage of children aged 6 months to 14 years who are moderately-severely anemic (low hemoglobin levels < 8 gldl) | Kenya MIS, 202034, UDHS, 201628 | |
| Malaria | Percentage of children aged 6 months to 14 years who tested positive for malaria by rapid diagnostic test | Kenya MIS, 202034, MOH, Uganda (2022) | |
| Helminths | Maximum point prevalence of schistosomiasis and soil transmitted helminth infections | Global Atlas of Helminth Infections35 | |
| Structural | Distance to the nearest healthcare facility | Percentage of women aged 15–49 years who reported they faced a problem of long distance to the health care facilities | KDHS, 20227 UDHS, 201628 |
| Postnatal care | Percentage of live births (newborns) aged 12–23 months who didn’t receive postnatal check within 2 months after birth. | KDHS, 20227 UDHS, 201628 | |
| Health Insurance | Percentage of households with no specific type of health insurance (National Health insurance fund, Private or community based) | KDHS, 20227, UDHS, 201628 | |
| Place of delivery | Percentage of live births who were not delivered at a health facility | KDHS, 20227 UDHS, 201628 | |
| Immunisation cards | Percentage of children 12–23 months who didn’t have vaccination card. | KDHS, 20227, UDHS, 201628 | |
| Rural population | Percentage of households living in rural areas | KDHS, 20227, UDHS, 201628 | |
| Social | Household wealth | Proportion of children aged 12–23 months born to poorer/poorest households (according to the DHS wealth quintile classification) | Kenya MIS, 202034 UDHS, 201628 |
| Maternal education | Percentage of women with low level of education (either primary or no education) | Kenya MIS, 202034, UDHS, 201628 | |
| Access to mass media | Percentage of women who had no access to specific media (newspaper, radio, TV) at least once in a week. | KDHS, 20227, UDHS, 201628 | |
| Access to safe and clean water | Proportion of households without access to improved water sources | Kenya MIS, 202034, UDHS, 201628 | |
| Housing conditions | Percentage of households living in informal dwellings | KCHSP, 202036 UNPS, 201837 | |
| Poor sanitation facilities | Percentage of households with unimproved sanitation facilities | Kenya MIS, 202034, UDHS, 201628 | |
| Transport means | Percentage of households with only non-motorised means of transport to the nearest health facility. Non-motorised includes animal-drawn cart, bicycle, boat without a motor and walking. | Kenya MIS, 202034, UDHS, 201628 |
Biological vulnerability measures susceptibility of individuals or populations to experiencing suboptimal vaccine induced immune responses24. Individuals who are biologically vulnerable may exhibit shorter duration of protection or weakened immune responses, leading to reduced vaccine efficacy and increased susceptibility to VPDs. Previous studies, including a meta-analysis of the effects of infections14, a review of nutritional factors15 and a comprehensive review13, have investigated factors that influence vaccine immunogenicity. In this study, we focus on modifiable biological factors common in African settings such as malnutrition, and parasitic infections, for which data are readily available.
Malnutrition
Malnutrition is a condition where a person`s nutrients or energy levels is deficient, excessive or imbalanced25. It can manifest in different forms including underweight, overweight and micronutrient deficiencies (lack of important vitamins and trace minerals)25. For purposes of this work, the focus is on common population level measure of malnutrition in Africa, specifically stunting (a measure of underweight), and Anaemia prevalence, form of iron deficiency. Malnutrition accounts for nearly 45% of child mortality in Africa26. Stunting affects approximately 165 million children under five in Africa27, with 26%28 and 29%7 prevalence in Uganda and Kenya, respectively. Nearly half (42.6%)7 of the children in Kenya are anaemic, compared to a lower prevalence of 29.4%28 in Uganda. Malnutrition is likely to lead to immune deficiencies, which may adversely affect the quality of vaccine immune responses. For instance, a review study found out that, malnourished children tend to exhibit lower sero-protection and reduced efficacy for measles and rotavirus vaccines; however, data regarding other vaccines such as BCG and Hepatitis B remains inconclusive29. Furthermore, iron deficient children at the time of vaccination in Kenya showed reduced vaccine response to diphtheria, pertussis, and measles vaccines30.
Exposure to infections
Parasitic infections such as helminths, malaria, and cytomegalovirus (CMV) are associated with impaired vaccine responses14,31,32. Many African populations, particularly young children and pregnant mothers are heavily exposed to these infections, due to poor access to clean water, inadequate sanitation facilities, poor housing conditions, and high levels of poverty33. Helminths are highly prevalent in many African countries. For instance, schistosomiasis prevalence among districts in Uganda ranges from 7.2% to 88.6%38 and 2.1% to 18% among Kenyan preschool children33. Helminth infections stimulate the production of regulatory T cells, which suppress inflammation and modulate the immune system to tolerate the parasite in the host’s body. This mechanism can weaken the body’s ability to mount strong immune responses to vaccines14,24,39,40. Additionally, chronic parasitic infections, like soil-transmitted helminths, are associated with stunted growth, anemia and micronutrients deficiencies, impacting immune function and vaccine effectiveness41,42. Similarly, malaria has been shown to reduce antibody production and long lasting immunity through immune dysregulation and immunosuppression, for instance a study in Uganda found decreased measles vaccine antibody responses in malaria exposed pregnant mothers and children, with similar results reported for BCG, tetanus, and pneumococcal vaccines43–46.
Therefore, based on the availability of data in Uganda and Kenya, the following indicators of biological vulnerability were chosen, prevalence of stunting, prevalence of anemia, and prevalence of malaria (Table 1).
The social vulnerability domain includes social, cultural, and economic factors influencing vaccine access and acceptance. Several individual factors contribute to low vaccination rates in some parts of Africa, with maternal education and income levels being the most significant8,47,48. In Uganda, 54% of women have low education levels28, compared to 49% in Kenya7, limiting their knowledge on benefits of vaccines, vaccination schedules, and potential risks of VPDs. This may result in vaccine hesitancy and poor decision making. Low income also affects vaccine uptake due to financial barriers such as transportation costs to the immunisation centers even when vaccines are free. Community related factors such as access to mass media, poor living conditions, also play a critical role in influencing vaccine impact9. Limited access to reliable media sources can amplify misinformation and vaccine hesitancy, as seen with COVID-19 vaccine skepticism49–51. Poor living conditions such as poor housing structure, lack of clean water, and poor sanitation facilities, may negatively mediate the biological factors that influence vaccine efficacy. These conditions are prevalent in African countries, for instance, in Uganda, 24% of households had structures with poor roofing materials and about 21% lacked improved water sources in 2019. In Kenya, about 55% of the population lives in informal settlements52. Such conditions facilitate disease transmission (e.g. tuberculosis, COVID-19, and malaria)53,54 and increase exposure to pathogens through contaminated food and water, hindering vaccine efficacy. For example, a trial among Zimbabwean infants showed that infants exposed to poor water, sanitation and hygiene (WASH) had reduced immune responses to rotavirus, evidenced by lower antibody levels and reduced seroconversion rates55.
The following indicators of social vulnerability were therefore chosen: demographic factors, community factors such as poor living conditions, limited access to mass media, and possession of non-motorized means of transport (Table 1).
Structural vulnerability includes physical, logistical, institutional, and policy related conditions that can affect delivery, distribution, accessibility, and quality of the immunisation services9,56,57. These factors are often external to the individual and operate at various levels including community, healthcare system, and national levels. Examples include: healthcare infrastructure, staff and training, policies and governance, and supply chain and logistics9,56,57. These factors may contribute to reduced vaccine impact by creating barriers to vaccine access and uptake. For instance, many African countries, vaccine availability is still a challenge in low income and middle countries. In Kenya, about 62.7% of the health facilities in Tana River County reported routine vaccine shortages in 202058. Similarly, in Homia Uganda, facilities also experienced vaccine stockouts57. The lack of vaccines at the facilities has been significantly associated with low vaccine coverage. For example in Nigeria, mothers reported making multiple visits to the health facilities on several occasions which was costly and time consuming and didn’t find the vaccines there, which may discourage and likely lead to incomplete immunisation for their children9. Furthermore, geographic location including proximity to the nearest health facility, transportations and availability of reliable transport means were significant with a child being immunized9,56,57. For instance, in Turkana, Kenya, 38.6% of the households reported that their travel time to the nearest health facility was greater than two hours7. This may account for the relatively low vaccine coverage in Turkana of, about 60%. Similarly, findings from a qualitative study revealed that the key structural factors facilitating uptake of COVID-19 vaccine among the elder persons were long distances to the vaccination sites, vaccine stockouts, and long waiting lines at the vaccination centres59. Thus, the following indicators of structural vulnerability were chosen distance to the healthcare facilities, access to postnatal care services, health insurance coverage, place of delivery, possession of immunization cards, and rural population (Table 1).
In this study, we used secondary data mainly obtained from national household surveys such as demographic health surveys which provide nationwide population level information that would be difficult and costly to collect prospectively across multiple districts or countries in Uganda and Kenya. In addition, these surveys are nationally representative, cover large sample sizes and provide data disaggregated at a first and second administrative units, allowing district level analysis. Importantly, they provide extensive data on many of the social, structural, and biological indicators relevant to vaccine impact, as well as vaccine coverage data. Data for social, structural and biological vulnerability indicators influencing vaccine impact were obtained from national household surveys, including Uganda demographic health survey (UDHS,2016)28 and Kenya Demographic Health survey (KDHS, 2022)7. Malaria prevalence data for Uganda were obtained from the Ministry of Health, while for Kenya, malaria data were drawn from the Malaria Indicator survey (MIS,2020) report34.
Immunisation program performance data for Uganda (January–December 2022) was sourced from the Ministry of Health. Vaccine coverage was defined as the proportion of children under one year receiving measles-containing vaccine (MR1), and the first (DPT1) and third (DPT3) doses of pentavalent vaccine. Coverage estimates were based on administrative data collected through the Reach Every District (RED) strategy, available for 146 districts. However, only 123 districts were included in the analysis due to missing data in newly created districts not captured in the 2016 UDHS. Coverage estimates exceeding 100% were capped at 100% for mapping. In Kenya, vaccine coverage data were obtained from the KDHS (2022) and defined as the proportion of children aged 12–23 months receiving all basic antigens, including BCG, OPV/IPV, DPT-Hib-HepB, and MR17. Differences in vaccine coverage definitions reflect variations in data sources and available indicators across the two countries. Helminth prevalence was excluded from index construction due to incomplete data across districts and counties. All datasets were linked to district/county shapefiles for spatial analysis.
Data on the immunization program performance in Uganda for a period of 12 months from January to December 2022 were obtained from the Ministry of Health (MoH). Vaccine coverage was defined as the proportion of children under one year who have received three basic vaccines: first dose of measles (MR1), first (DPT1) and third (DPT3) dose of pentavalent vaccine.
Vaccine coverage data was obtained for all 146 districts, including the new districts. For Kenya, immunization data was obtained from the Kenya Demographic Health survey (KDHS,2022)7. Vaccine coverage was defined as the proportion of children aged 12–23 months receiving all the basic antigens; BCG vaccine; three doses of polio (OPV, IPV); three doses of DTwP-Hib-HepB; single dose of Measles-Rubella (MR). The variation in the definitions for vaccine coverage was due to the different data sources and the data available for specific vaccines. In Uganda, vaccine coverage was calculated using administrative data collected as part of the Red Every District (RED) strategy, used to evaluate immunisation performance for each district. This approach focuses on coverage for measles (MR1), DPT1 and DPT3, that are routine tracked through health records and are available for most districts. On the other hand, in Kenya, household survey data was used, which includes a wide range of antigens. Data for risk factors for impaired vaccine impact was obtained from household surveys, including demographic health survey (DHS) conducted in Uganda (2016) and Kenya (2022). Data on malaria prevalence was obtained from the Malaria Indicator survey (MIS) reports and ministry of health (Table 1). Newly created districts in Uganda (beyond the 123 defined in the 2016 UDHS) were excluded due to incomplete data. In the construction of the index, we excluded helminths prevalence as the data was not consistently available for all counties and districts. The data was then linked to the shape files for spatial analysis.
The CVIVI was constructed in four steps, namely, normalization, percentile rank calculation, weighting, and aggregation (Figure 1).
Indicator values were scaled between 0 (least vulnerable) to 1 (most vulnerable) using the max-min approach60,61 to standardize the disparate data scales.
Where yin is the normalized indicator and Xin is the indicator value
The percentile rank for all the selected indicators presented in Table 1 was calculated. The percentile rank methodology has been commonly employed in defining vulnerability indices related to infectious diseases such as COVID-1962 and climate change63. Each indicator was ranked in ascending order such that higher values indicate greater hypothesised vulnerability. Districts or counties were assigned ranks, and the percentile rank for all the selected indicators described in Table 1 was computed using the formula:
where rij is rank of indicator j, in district/county i, Nj is the number of districts/counties with indicator j and Prij represents the percentile rank of indictor j, in district/county i. The percentile rank is a statistical measure ranking each data point in relation to the full dataset (for instance 40th percentile represents the value below which 40% of the data falls). In this context, higher percentile rank values denote higher vulnerability (Prij = 1.0), while lower values represent lower vulnerability (Prij = 0.0).
The final vulnerability index calculation is determined by the choice of the weights, and there are several ways to determine these weights including statistical methods such as principal component analysis (PCA), factor analysis, equal weights, and participatory approaches such as Analytic Hierarchy Process (AHP)60,61. In this study, we explored principal component analysis (PCA), regression-based weights and equal weighting. To assess suitability for PCA, we used the Kaiser-Meyer-Olkin (KMO) test63. The KMO test shown fair suitability for Uganda (KMO= 0.74), but Kenya had poor adequacy (KMO=0.5), indicating that PCA derived weights would be unreliable and not comparable across Uganda and Kenya. Regression-based weights were also not feasible due to limited and inconsistent outcome data on vaccine coverage and lack of data on immune response. To avoid bias and ensure comparability, we adopted an equal weight approach. In this approach, indicators were equally weighted within each domain, and the three domains (social, structural, and biological) were then given equal weights in the composite vulnerability index. This method, while simple, provides a transparent and robust approach, and has been applied in index construction of established indices such as the Surgo Foundation Community COVID-19 vulnerability index64 and Centre for Disease Control and Prevention (CDC) social vulnerability index65.
The domain vulnerability of each district/county was obtained by summing the percentile ranks for all indicators in each specific domain, given as
where k represents the number of domains i.e. k = 1, 2, 3 as shown in Table 1; DVik represents the vulnerability value of district/county i computed based on indictors in domain, k, and n is the number of indicators in each domain. The Community Vaccine Impact Vulnerability Index, CVIVIi for each district/county was calculated as the average of domain-specific scores as follows:
For spatial mapping, we firstly harmonized district and county names across the indicator datasets and shape files. CVIVI scores were categorized into five vulnerability classes i.e. least, less, moderate, more, and most based on the relative vulnerability of each district or county. Classification was performed using the quantile classification method in QGIS, ensuring each class contained an equal number of geographic units66. For easy interpretation, CVIVI scores were normalized on a 0–100 scale, where higher values denote greater vulnerability to reduced vaccine impact, and lower values, low vulnerability. It is important to note that the CVIVI reflects relative vulnerability across districts or counties; a score of 0 does not imply the absence of vulnerability, but rather the lowest observed value compared to scores to other districts/counties. To explore spatial relationships between vaccine coverage and vulnerability, bivariate maps were created by overlaying CVIVI scores with vaccine coverage data. Both variables were divided into tertiles (high, moderate, and low) to visualize co-distribution patterns and highlight areas of mismatch such as high vulnerability coinciding with low coverage.
For easy interpretation of the results, the developed index values were normalized in the range of zero to one where zero indicates least vulnerability and 1 indicates very risk to reduced vaccine impact. It’s important to note that the CVIVI measures the relative levels of vulnerability to reduced vaccine impact among districts or counties, which means that an index of value 0.0 doesn’t imply absence of vulnerability to reduced vaccine impact within the district or county.
The correlation heatmaps in Figure 2 and Figure 3 illustrate the pairwise relationships between vulnerability indicators themselves, as well as their relationship with vaccine coverage in Kenya and Uganda, respectively. Each cell represents the correlation coefficient between two variables, with positive correlations shown in shades of blue and negative correlations in shades of red.
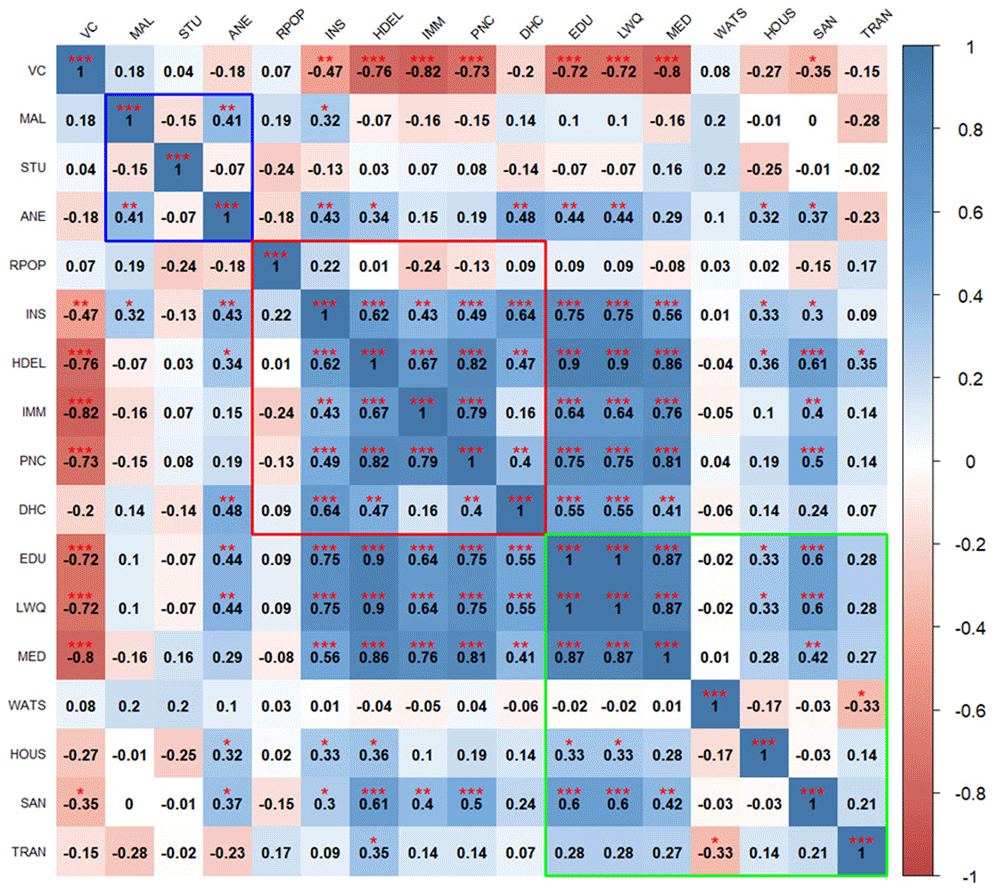
Blue outline represents biological factors, red outline represents structural factors, and green outline represents social factors. Asterisks indicate level of significance: ***p value < 0.001, **p value < 0.01, *p value < 0.05.
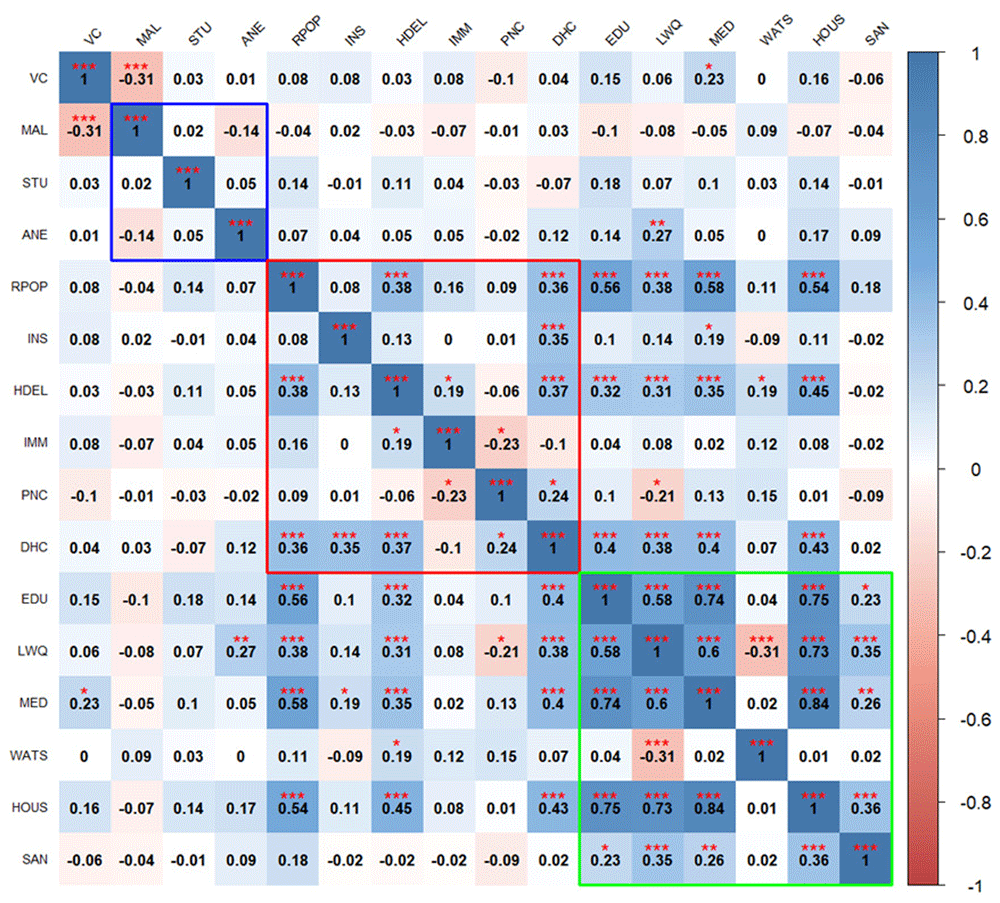
Blue outline represents biological factors, red outline represents structural factors, and green outline represents social factors. Asterisks indicate level of significance: ***p value < 0.001, **p value < 0.01, *p value < 0.05. The acronyms used in the Figure 3 and Figure 4 are as follows: VC (Vaccine Coverage), MAL (Malaria prevalence), STU (Stunting prevalence), ANE (Anemia prevalence), RPOP (Rural population), INS (Insurance coverage), HDEL (Home delivery), IMM (No immunization card), PNC (No postnatal care for newborns), DHC (Long distance to nearby health facility), EDU (Low mother’s education level), LWQ (Low wealth quintile), MED (Limited access to mass media), WATS (Access to unimproved water sources), HOUS (Poor housing structures), SAN (Access to unimproved sanitation facilities), and TRAN (Ownership of non-motorized transport means).
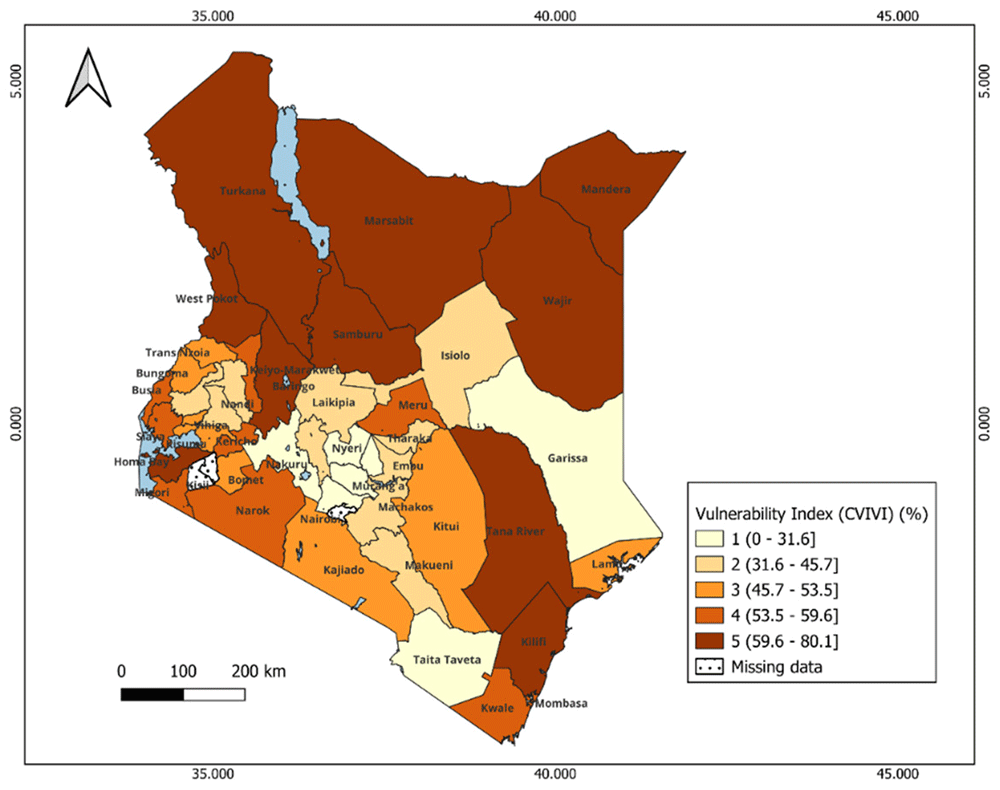
Dark colors represent high vulnerability, and light colors correspond to low score vulnerability. Scores are categorized by groups: 1= least vulnerability, 2 = less vulnerable, 3 = moderately vulnerable 4= More vulnerable, 5= most vulnerable.
In Kenya, there is significant negative correlation between vaccine coverage and structural factors such as high home-based deliveries, lack of immunization cards, no postnatal care for newborns, and social factors such as low maternal education, low family income, and limited access to media. No consistent patterns of correlation were seen between vaccine coverage and biological factors, with positive and negative correlations observed within this domain. For example, anemia is positively correlated with malaria, while stunting is negatively associated with malaria.
Biological indicators show weak or inconsistent correlations with social and structural indicators, though some notable relationships exist, for instance, higher anemia prevalence is linked to home deliveries and lack of insurance coverage. Structural indicators are positively correlated with each other except for the percentage of rural population. Structural factors significantly correlate with most social factors, particularly maternal education, income level, and access to media. Social indicators are strongly correlated with each other.
In Uganda, the findings show that vaccine coverage is negatively correlated with high malaria prevalence (Figure 3). Also, vaccine coverage is poorly correlated with all the structural factors. Access to media stands out as the only social factor positively correlated with vaccine coverage.
Biological factors exhibit weak correlation with each other and with indicators in other domains, except, anemia prevalence, which correlates positively with low wealth quintile. Within the structural domain, few indicators show significant positive correlations with others such as rural population, home based deliveries, long distance to the health facilities showing positive weak correlations while lack of postnatal care exhibits negative correlations. Social factors are generally positively correlated with each other, except for limited access to improved source water. Rural population and home-based deliveries positively correlate with low maternal education, low income, limited access to media access and poor housing structures.
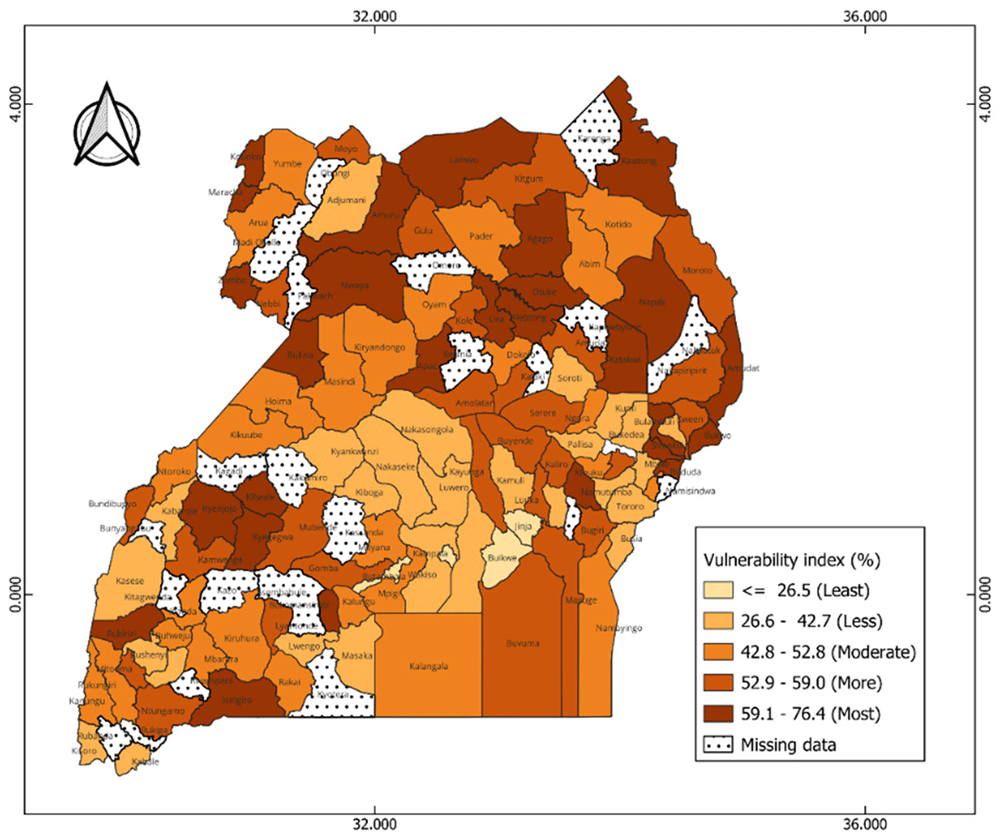
The index reveals significant geographical patterns in the vulnerability levels between and within each country, as shown in the maps (Figure 4 and Figure 6) and Extended data, Tables 1 & 2 in Kenya and Uganda, respectively. The dark colors indicate districts at a high risk of reduced vaccine impact, indicating that communities in these areas are less likely to benefit from vaccines. Bar plots 5 and 7 present the average estimates of key indicators across each vulnerability group, helping us identify the main factors contributing to vulnerability in each group. The contributing factors were those with relatively higher values as compared to other areas (see Extended Data Tables 4 & 5).
In Kenya, most vulnerable counties form clusters within specific regions. For instance, most vulnerable counties include Mandera, Turkana, Garissa in the eastern, West Pokot, in the western and Kilifi and Tana River in the southern region. These counties face a combination of social, structural and biological challenges such as the high prevalence of anemia, lack of immunisation cards, low maternal education, long distance to the near health facility, limited access to post-natal care services for newborns, low wealth quintile, and limited access to mass media (Figure 5). Despite these challenges, malaria prevalence in these counties is relatively lower than compared to other counties (see Extended data, Table 4). Counties with moderate vulnerability are primarily located in the central and southern parts of Kenya. Examples of these counties include: Kwale, Meru, Kajiado, and Narok. Conversely, counties in the central region (e.g., Kiambu, Nyeri, Machakos) and Taita Taveta (south) show low vulnerability scores (Figure 4). These counties benefit from better healthcare access and high levels of maternal education, which is likely to contribute to the low prevalence of biological indicators such as malaria and stunting (see Extended data, Table 4). Notably, health insurance coverage remains relatively low across all counties, regardless of their vulnerability level. Also, most of the households had poor access to unimproved and shared toilets.
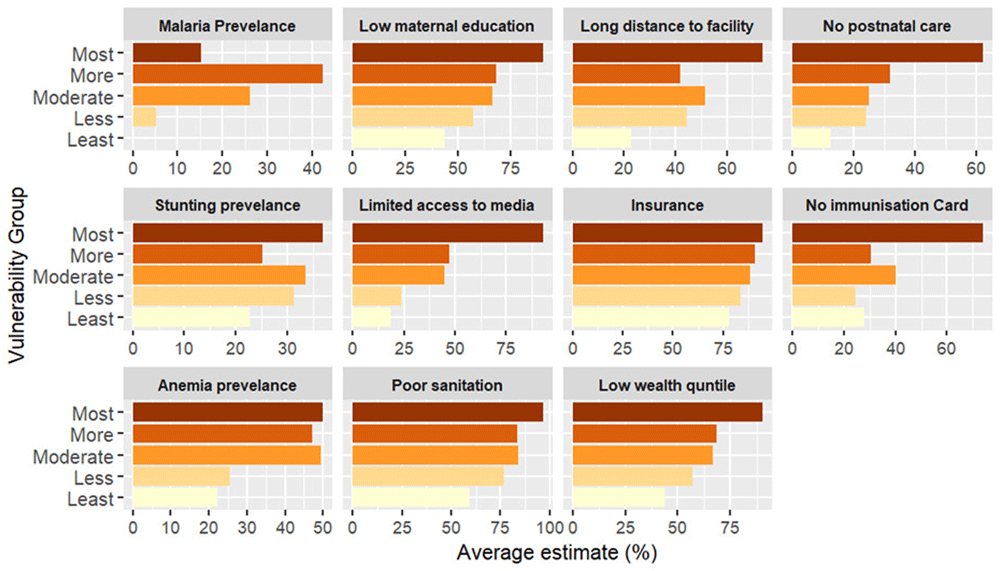
The figure presents the average estimate of key indicators across vulnerability groups, from the most vulnerable (dark shades) to the least vulnerable (light shades). Each panel represents a specific indicator, illustrating its contribution to community vulnerability.
The index scores are grouped onto 5 groups with the dark colors representing high vulnerability scores, and light colors correspond to low score vulnerability scores.
In Uganda, vulnerable districts are scattered across all regions. For instance, most vulnerable districts such as Amudat and lamwo are in the northern region, Buliisa and Kyenjojo in the western region, and Bulambuli and Bududa in the eastern region (Figure 6). Communities in these districts are faced with significant challenges including high stunting prevalence, high levels of poverty, limited access to postnatal care services for newborns, low maternal education levels, and limited access to mass media (Figure 7). Moderately vulnerable districts are also observed across different regions such as Kotido and Abim in the north, Namayingo and Ngora in the east, and Mpigi and Kayunga in the central. The least vulnerable districts are mainly concentrated in the central region such as Kampala, Buikwe, and Butambala. Despite their low vulnerability, these areas still face some challenges. For instance, Kampala, an urban district is characterized by high malaria incidence (218.5 cases per 1000 population), low insurance coverage (96.3%), limited access to postnatal care services (68.4%), and limited access to mass media by women (55.8%) (see Extended data, Table 5).
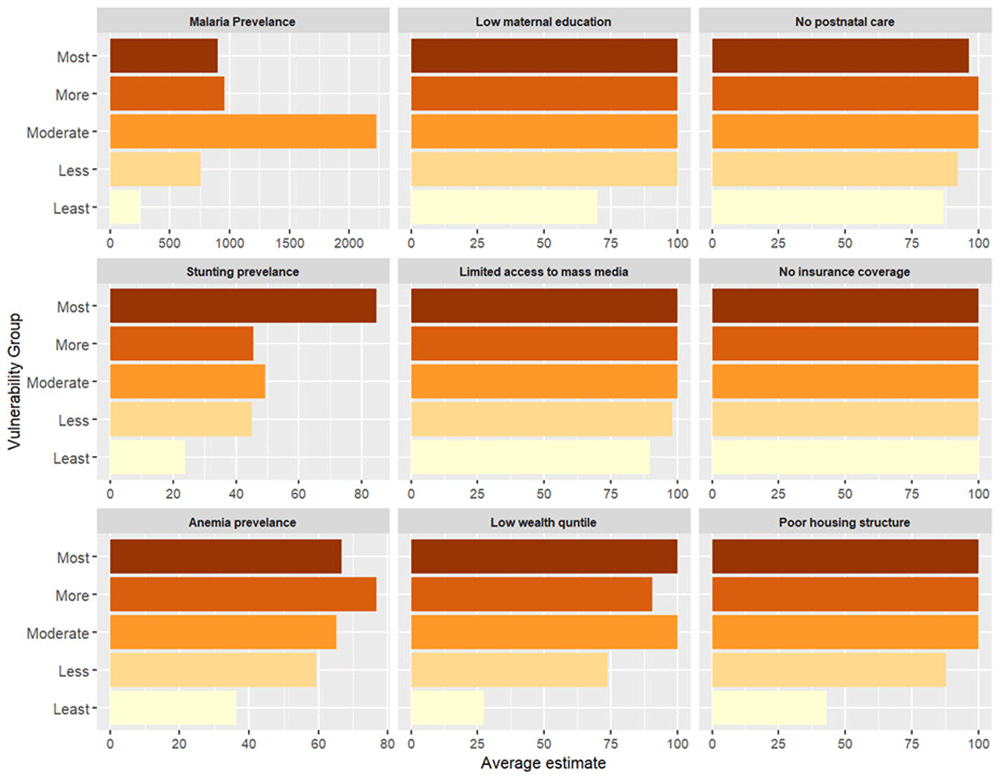
The figure presents the average estimate of key indicators across vulnerability groups, from the most vulnerable (dark shades) to the least vulnerable (light shades). Each panel represents a specific indicator, illustrating its contribution to community vulnerability.
Buliisa is in southwestern Uganda, ranked most vulnerable district in Uganda, with a CVIVI score of 76% (Figure 4, and Extended data, Table 1). The district is characterized by high levels of poverty, low literacy levels and limited access to essential services. According to the UDHS (2016), majority of mothers either have no or low formal education and have no access to mass media like television, radios, contributing to low awareness and uptake of vaccination services (see Extended data, Table 4). In addition, 80% of mothers lacked postnatal care, and many households are located over 5 km from a health facility. The district also reported high rates of anaemia (58.3%) and high malaria incidence reported at 600 cases per 10,000 population in 2022 (see Extended data, Table 4). Routine vaccine coverage (DPT1, DPT3, measles) averaged 69.7% in 2022. Buliisa district experienced a yellow fever outbreak in 2024 (13 cases, 1 death) and recurrent measles outbreaks.
Turkana, in north-western Kenya, had the highest vulnerability score (77.3%) (Figure 5, Extended data, table 2). The county faces high poverty, low maternal education (<10% with secondary education), and limited health infrastructure. Over 30% of children are stunted (see Extended data, Table 5). Vaccine coverage for basic antigens was reported at 60.1% in 2022. In 2023, Kenya experiences measles outbreaks in several counties, among which Turkana, which reported the highest number of (582 cases), accounting for about 38% of national total67.
The overall CVIVI is a composite score that may obscure specific challenges. Districts or counties with low CVIVI scores may still exhibit high scores in at least one domain. Thus, dis-aggregating the index into structural, social and biological domain scores reveals distinct geographical patterns as shown in spatial maps in Extended data, Figure 1 & 2 and Extended data, Table 1 & 2
In Kenya, social vulnerability is highest in northern and coastal counties (see Extended data, Figure 1- panel A), driven by limited access to mass media, poor sanitation and low maternal education levels (see Extended data, Table 5). Mandera county exhibits the highest prevalence of these barriers. In Marsabit county, 33.4% of the households lack improved water sources, while 92.3% lack access to proper sanitation facilities. Moderately vulnerable counties, such as Isiolo, Kitui, and Busia have better access to mass media and improved water sources, balancing their overall vulnerabilities. Conversely, counties in central and southwest Kenya show low vulnerability due to higher maternal education levels, better media access and low poverty levels, though they struggle with inadequate sanitation facilities, with toilet facilities often shared.
In Uganda, the most socially vulnerable districts are concentrated in the northern region, with a few in the southwest (see Extended data, Figure 2-panel D) characterized by high poverty, low mothers' education, limited access to mass media, poor housing conditions, and unimproved sanitation facilities, despite better access to improved water sources. Districts in the Central region are least vulnerable, with relatively high-income levels and better infrastructures, though women still face challenges with limited media access.
In Kenya, high structural vulnerability was observed among counties predominantly situated in northern and southeast Kenya (see Extended data, Figure 1-panel B), driven by limited insurance coverage, reliance on non-motorized transportation, and limited access to postnatal care for newborns. Conversely, the less vulnerable counties like Kericho, Nandi, and Kajiado, are characterized by improved healthcare services, and improved means of transport. However, structural challenges, such as high rates of uninsured households (e.g. 82% in Nyeri), persisted even in less vulnerable communities (see Extended data, Table 4).
In Uganda, structural vulnerability was unevenly distributed, with the highest scores in the northern districts and a few districts in the southwest (see Extended data, Figure 2-panel E). These areas are predominantly rural, facing challenges such as low insurance coverage, home deliveries, and limited access to postnatal services. Strikingly like the Kenyan scenario, despite their vulnerability, these districts exhibit a higher proportion of children with immunization cards. Moderately vulnerable districts in central and southwestern Uganda (e.g. Hoima, Kikuube, and Masaka) exhibited mixed outcomes. While these areas demonstrate positive indicators like the high prevalence of health facility deliveries and access to postnatal care services, household insurance coverage remains a significant challenge. Urban districts like Kampala and Wakiso districts were the least vulnerable due to better access to healthcare services and widespread immunisation services, though gaps in access to postnatal services and insurance coverage persist.
In Kenya, counties located in the coastal areas (e.g. Kilifi) and the southwest region (such as Turkana and Tana River) display high biological vulnerability due to high prevalence of stunting and anemia among children aged 6–35 months (see Extended data, Figure 1-panel C). Additionally, malaria prevalence is particularly high in some counties such as Busia and Kisumu, due to their low-lying and humid environment. The least vulnerable counties are in the central region of Kenya, such as Samburu, Baringo, and Kiambu, as well as Taita Taveta in the southern region of Kenya. In Uganda, the results show that different biological vulnerability levels are dispersed across the country (see Extended data, Figure 2-panel F), emphasizing the heterogenous nature of the biological factors. Districts such as Koboko, Nakapiripit, and Lira situated in the northern region and water-body proximate districts such as Buliisa and Kalangala exhibit high vulnerability, primarily attributed to high prevalence of malnutrition, and high malaria prevalence, respectively.
Our analysis reveals that communities with identical overall vulnerability scores can exhibit markedly different vulnerability profiles across domains. To illustrate this, we compared two counties in Kenya: Wajir and Kilifi counties. These have nearly identical overall CVIVI scores (65.4% and 66.2%, respectively) but with different domain-specific vulnerability scores (Figure 8).
In Wajir, structural factors are primary drivers of vulnerability, with the county scoring 79 on this domain. Most households in Wajir reported facing long distances to the nearest health facilities, and 62.2% of women did not receive postnatal care for their newborn infants (see Extended data Table 468). Wajir is one of the counties with low vaccine coverage (48.6%). On the other hand, biological and social factors are the main vulnerability drivers in Kilifi scoring 77% and 73%, respectively. Biological vulnerability is largely attributed to the high prevalence of anaemia among children (45%), which is relatively high when compared with other counties in Kenya. Social vulnerability is characterised by high poverty levels (69%), low maternal education (69%), and poor sanitation (80%). Kilifi exhibits a relatively high vaccine coverage (89.8%).
In Kenya, significant variations in vaccine coverage are observed across counties. The lowest vaccine coverage is reported in counties boarding Somalia, such as Garissa and Mandera, ranging from 20% to 40% (Figure 9). Counties with highest vaccine coverage are located mainly in the central region, with vaccine coverage estimates ranging from 79% to 96%, and Vihiga having the highest percentage (96%).
In Uganda, many districts (72 out of 145) reported vaccine coverage greater than 100%, while only one district has missing data. The map (Figure 10) shows less geographical heterogeneity in the spatial distribution of vaccine coverage across districts, especially when compared to Kenya. Most of the districts in the north and central regions have relatively high vaccine coverage except districts in hard-to-reach areas like Nakapirit (74.3%) and some rural districts like Buliisa (69.3%). Wakiso has the lowest vaccination coverage (40.8%).
Correlation between vaccine coverage and vulnerability index
In Kenya, a negative correlation (R= -0.53, p < 0.001) is observed between vaccine coverage and CVIVI score (Figure 11, panel A), indicating that counties with lower vaccine coverage tend to have higher CVIVI scores. These findings are illustrated in the bivariate map, which shows clustering of high vulnerability scores in areas with low vaccine coverage (see Extended data, Figure 3). For example, northwest counties like Turkana, West Pokot, and Mandera, as well as coastal regions such as Tana River, exhibit both high CVIVI and low vaccine coverage. Conversely, counties in the central region demonstrate high vaccine coverage and low CVIVI scores. Interesting, Garissa, which had the lowest vaccine coverage also has the lowest CVIVI score, suggesting that the factors considered may not explain the low vaccine coverage in this county. In Uganda, there is no correlation between CVIVI and vaccine coverage (R = 0.009, p = 0.925) (Figure 11, panel B). Bivariate maps (see Extended data, Figure 4) highlight a small number of central districts, such as Kampala and Wakiso, which have low CVIVI scores and high vaccine coverage. Notably, Buliisa stands out as the only district with both high CVIVI and low vaccine coverage.
In this study, we developed a community vulnerability index to identify communities in Uganda and Kenya at a risk of reduced vaccine impact based on underlying community’s structural, social and biological factors influencing vaccine impact. Our findings reveal distinct patterns in the geographic distribution of vulnerability within and between the two countries. In Kenya, different vulnerability levels were clustered in specific regions, with the most vulnerable counties mainly found in the northwest and southeast of Kenya including Turkana, Mandera, and West Polot counties. These findings are consistent with patterns observed in the COVID-19 Social Vulnerability Index (SVI) developed during the pandemic, which also identified sub counties in the northwest and some in eastern Kenya as most socially vulnerable areas due to high levels of poverty, poor access to healthcare services, and low levels of education69. In Uganda, vulnerability was more scattered, with the most districts concentrated in the northeast (such as Amudat, Lamwo) and southwest (such as Buliisa and Kyenjojo). These areas primarily comprise of rural communities, geographically isolated regions, and some have a significant number of refugees, particularly in northern Uganda70. In addition, some of these vulnerable districts have experienced severe disease outbreaks. For example, between 2020 and 2023, 144 confirmed measles outbreaks occurred across 29 districts. Among these, Lamwo, one of the most vulnerable districts, reported 28 laboratory confirmed measles cases. The recurring outbreaks indicates gaps in vaccine coverage and population immunity despite ongoing routine and measles campaigns in these districts71. These spatial patterns align with findings from the zero-dose vulnerability index, which identified several high-risk districts in northern and eastern Uganda including Kabongo and Amudat as hotspots for unvaccinated children22. The contributing factors to high vulnerabilities in these areas are shown to cut across various domains, indicating multiple interrelated factors may contribute to reduced vaccine impact22. In both countries, the most prevalent structural factors were; long distance to the nearest health facility, and lack of postnatal care for newborns. Among social factors, low maternal education, and poor households were prominent, while for biological factors, the prevalence of stunting and anemia was relatively high. Additionally, challenges such as limited access to health insurance were highly prevalent even within less vulnerable communities. These findings are consistent findings from spatial analysis in Uganda and Kenya, which shown that long travel times, limited media access and rural residence significantly increased risk of being under or no vaccination5,72. Similarly, findings from a multidimensional household vulnerability index in Nigeria shown that a combination of demographic, social, geographical, and economic factors were associated with increased risk of partial or no vaccination73. While these studies primarily focused on identifying geographic hotspots of unvaccinated or under vaccinated children and the associated determinants of vaccine coverage, the CVIVI extends this body of literature by focusing on vulnerability to reduced vaccine impact, not just vaccine coverage gaps. By integrating a range of domain specific risk factors, including biological factors, the CVIVI provides insights on why vaccine impact may be suboptimal in communities with reported high vaccine coverage. In addition to mapping vulnerable communities, our analysis further reveals heterogenous community vulnerability profiles even with identical overall vulnerability scores, with each district/county vulnerable at least in one domain. This suggests that different communities face unique challenges implying that universal solutions may not effectively address the health disparities associated with these risk factors within these communities, potentially leading to impaired vaccine impact. Tailored, context-specific interventions are needed to address distinct challenges faced by each community. These findings highlight the need for cross-sector collaborations between health, education, and infrastructure sectors to improve vaccine benefits and promote health equity in these vulnerable community. The findings also demonstrate negative correlation between the vulnerability index and vaccine coverage, particularly in Kenya where heterogeneity in vaccine coverage was observed. However, in Uganda, despite notable geographic disparities in the index, no correlation between the vulnerability index was observed. This is likely due to the uniform distribution of the vaccine coverage estimates across districts as well as data quality issues74. This highlights that relying solely on vaccine coverage estimates to evaluate vaccine impact vulnerability and identify vulnerable communities may not be an effective approach. Nevertheless, the CVIVI has the potential to identify vulnerable communities in situations where poor quality or unreliable data on vaccine coverage exists.
This study has some limitations. The study relied on secondary analysis of existing datasets that may have gaps, inconsistencies and outdated information, particularly for data from 2016 Uganda Demographic Health survey. Inconsistencies in the vaccine coverage data for Uganda were observed, with some district-level estimates (72 out of 145) exceeding 100% due to denominator issues74. Additionally, differences in the definition of vaccine coverage used for Uganda and Kenya may also affect the comparability of vaccine findings between the two countries. Our analysis was based on aggregated data at district and county levels, which may mask heterogeneity within these large administrative units. While the index was useful for identifying communities, it doesn’t fully capture the interplay between social, structural and biological factor that contributes to reduced vaccine impact. Future work should consider conducting more fine-scale analyses such as household surveys, qualitative studies, and community engagement within vulnerable communities to fully understand vulnerability. Another key limitation is that the index has not been validated against empirical data. To address this, we plan to validate the index with the VANguard survey results to test how well the index aligns with the observed data and what it predicted. Additionally, we didn’t include data on health facility structural factors such as vaccine stockouts, vaccination staff availability, that would provide additional information on the supply chain and vaccine distribution challenges within districts or counties. Our analysis was limited to correlation and could not establish any casual relationships between the risk factors and vaccine impact. Finally, the equal weight scheme used in the index calculation requires further validation and sensitivity analysis to assess the impact of alternative weighting schemes. Despite these limitations, the study highlights the multidimensional nature of vulnerability and demonstrates that assessments based on multiple indicators provide a more holistic view than a single indicator or domain. By integrating data on social, structural and biological factors, our approach provides a more holistic assessment of vulnerabilities related to reduced vaccine impact. Thus, the index classification and mapping serve as a starting point for understanding how these factors interact to influence vaccine impact and identify where communities where targeted interventions are most needed. Additionally, the index is adapted to different spatial scales depending on the availability of data, making it’s a versatile tool for identifying vulnerable communities to guide efforts to improve vaccine impact in other settings.
The findings from this study have some policy implications for improving equity in immunisation programs. The Community Vaccine Impact Vulnerability Index (CVIVI) provides a potential evidence-based approach for identifying high risk areas where vaccine coverage estimates alone may not translate into vaccine impact or unreliable. For instance, in Uganda, several districts reported high vaccine coverage but likely vulnerable to reduced vaccine impact due to prevailing social, structural and biological challenges identified by the CVIVI. The CVIVI maps and scores can complement existing Reach Every district (RED) evaluation and planning tools to help national and subnational teams to prioritize high-risk areas during microplanning, routine outreach, and immunisation campaigns. This approach could inform allocation of resources and identify challenges even in areas with high reported vaccine coverage. Our findings also revealed that each district/county exhibited distinct vulnerability profiles highlighting that universal solutions may not improve community specific vaccine impact. The domain specific vulnerability scores can help identify specific reasons why a community may be at risk of reduced vaccine impact and inform design of tailored strategies. For example, districts facing structural barriers such as long distance to the health facilities may require expanded outreaches, or mobile vaccination teams mad community outreach programs to bridge access gaps75. In communities with social barriers like low maternal education and limited access to mass media, policy makers can prioritise targeted health education campaigns to improve vaccine confidence and maternal awareness76. In communities where biological vulnerabilities such as malnutrition and malaria are highly prevalent, integrated service delivery such as combining vaccination with deworming and micronutrient supplementation can improve on immune response and vaccine effectiveness77.
The CVIVI offers a potential practical framework for identifying and addressing inequities in vaccine impact across diverse communities. By integrating social, structural and biological factors that influence community level vulnerability, it provides comprehensive understanding of why some populations experience reduced vaccine impact even in areas with high reported vaccine coverage. In alignment with the WHO Immunization Agenda 2030 (IA2030), CVIVI findings support key policy priorities that can guide more inclusive and effective immunisation strategies. Firstly, the varying community vulnerability profiles support the need for community-tailored immunisation strategies that go beyond coverage to address underlying drivers such as maternal education, nutrition, and access to health services, addressing the IA2030’s goal of equity. Secondly, the overlapping vulnerabilities across structural, social and biological domains highlights the need for strong integration across immunisation, maternal health and child health services, nutrition programmes, community engagement, to maximize coverage and life course benefits. Together, these actions would ensure that no community is left behind in realizing the full benefits of vaccination.
We sought permission to use the KDHS and UDHS survey data from the MEASURE DHS program website https://www.dhsprogram.com/data/available-datasets.cfm. Ethical approval for the 2022 KDHS was granted by the Institutional Review Board of the Inner-City Fund (ICF). The survey was conducted by the Kenya National Bureau of Statistics in collaboration with other development partners. The protocol for the 2020 Kenya Malaria Indicator Survey (KMIS) was approved by the Kenyatta National Hospital/University of Nairobi Scientific and Ethics Review Committee and the institutional review board at ICF. Further details regarding the conduct of this survey may be found in the 2022 KDHS report7, and 2020 KMIS report34. Ethical approval for the 2016 UDHS was obtained from the Institutional Review Board of ICF and the Uganda National Council for Science and Technology (UNCST). The survey was implemented by the Uganda Bureau of Statistics (UBOS), in collaboration with Ministry of Health. Further details regarding the conduct of the study may be found in the 2016 UDHS report28. For all the surveys, written informed consent was obtained from all human participants and from legally appointed representatives of minor participants. No formal ethical approval was required for this work as it used secondary data from publicly available datasets.
All the data used in this work is publicly available via the data sources cited in the paper. Data on vulnerability indicators and vaccine coverage in Kenya were obtained from publicly available 2022 KDHS report7, and 2020 KMIS report34. In Uganda, data on social, structural and biological vulnerability indicators was obtained from the UDHS 2016 datasets, accessible on the DHS website https://dhsprogram.com/data/dataset_admin/index.cfm78. To access the data, permission was obtained from the DHS administration through registration and submission of a brief proposal for this study. Access to the datasets was granted within two working days. Immunisation data and malaria prevalence in Uganda were obtained through formal requests from the Uganda Ministry of Health. Shapefiles for Uganda and Kenya used for spatial analysis were freely downloaded from https://gadm.org/maps79
Open Science Framework: Assessing community vulnerability to reduced vaccine impact in Uganda and Kenya: A spatial data analysis. https://doi.org/10.17605/OSF.IO/QBYSJ68
The project contains the following underlying data:
Open Science framework: Assessing community vulnerability to reduced vaccine impact in Uganda and Kenya: A spatial data analysis. https://doi.org/10.17605/OSF.IO/QBYSJ68
The project contains the following extended data:
Supplementary material: Additional tables (Table 1: Domain specific and overall vulnerability index scores for counties in Kenya, Table 2: Domain-specific and overall vulnerability index scores for districts in Uganda, Table 3: Summary statistics of vulnerability indicators, Table 4: Estimates of vulnerability indicators across counties in Kenya, Table 5: Estimates of vulnerability indicators across districts in Uganda).
Supplementary material: Additional figures (Figure 1: Domain specific vulnerability scores across counties in Kenya, Figure 2: Domain specific vulnerability scores across districts in Uganda, Figure 3: Degree of correlation between the vulnerability index and vaccination coverage at the county level in Kenya, Figure 4: Degree of correlation between the vulnerability index and vaccination coverage at the district level in Uganda).
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC-BY 4.0)
All statistical analysis, and visualization was carried out in R software (version 4.4.2) available for free download at https://cran.r-project.org/. Geographical preprocessing and spatial maps were generated in Quantum Geographic Information System software (Q-GIS, version 3.38), which is also available for free download at https://www.qgis.org/.
The R code is available at the GitHub link:
https://github.com/Robinah23/Community-Vaccine-Impact-Vulnerability-Index-
RN was involved in conceptualization, methodology, data curation, formal analysis, writing of the original draft, data visualisation. AN and LZ were involved in methodology, formal analysis, reviewing and editing for the final draft of the manuscript. PC and HL contributed to data curation, resources and reviewing and editing. AE and PK were involved in funding acquisition, conceptualization, project administration, and reviewing and editing of the manuscript. CT and EW were involved conceptualization, methodology, supervision, writing and reviewing of the initial draft of the manuscript.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental Epidemiology, Health Economics, Pollution Control Management, Hydrogeochemistry, Groundwater Pollution, Groundwater Quality, Health Risk Assessment, Water Pollution and Management, Water and Gender, Chemicals & Health, Environmental Public Health Policy & Practice, Environmental Justice & Health Equity, Noise & Health, Carbon Accounting Services, Environmental & Health Impact Assessments, Community based participatory Research, Climate Change Mitigation and Adaptation, Oil Spills Clean up and Remediation, Environmental Toxicology & Health, Emissions Control and Respiratory Protection, Biogeochemistry, Earth sciences - Atmospheric sciences, Earth sciences - Climate change, Earth sciences – Limnology, Health care - Health education, Health care - Health policy, Health care - Health services research, Pollution research and control, Public health and epidemiology - Biostatistics and methodsPublic health and epidemiology - Chronic diseases, Public health and epidemiology - Environmental health, Public health and epidemiology – General, Public health and epidemiology - Global health, Public health and epidemiology - Health behavior, health promotion and society, Public health and epidemiology - Health policies, systems and management, Public health and epidemiology - Occupational health
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious diseases epidemiology; vaccines; social health inequalities; scientific police
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental Epidemiology, Health Economics, Pollution Control Management, Hydrogeochemistry, Groundwater Pollution, Groundwater Quality, Health Risk Assessment, Water Pollution and Management, Water and Gender, Chemicals & Health, Environmental Public Health Policy & Practice, Environmental Justice & Health Equity, Noise & Health, Carbon Accounting Services, Environmental & Health Impact Assessments, Community based participatory Research, Climate Change Mitigation and Adaptation, Oil Spills Clean up and Remediation, Environmental Toxicology & Health, Emissions Control and Respiratory Protection, Biogeochemistry, Earth sciences - Atmospheric sciences, Earth sciences - Climate change, Earth sciences – Limnology, Health care - Health education, Health care - Health policy, Health care - Health services research, Pollution research and control, Public health and epidemiology - Biostatistics and methodsPublic health and epidemiology - Chronic diseases, Public health and epidemiology - Environmental health, Public health and epidemiology – General, Public health and epidemiology - Global health, Public health and epidemiology - Health behavior, health promotion and society, Public health and epidemiology - Health policies, systems and management, Public health and epidemiology - Occupational health
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental Epidemiology, Health Economics, Pollution Control Management, Hydrogeochemistry, Groundwater Pollution, Groundwater Quality, Health Risk Assessment, Water Pollution and Management, Water and Gender, Chemicals & Health, Environmental Public Health Policy & Practice, Environmental Justice & Health Equity, Noise & Health, Carbon Accounting Services, Environmental & Health Impact Assessments, Community based participatory Research, Climate Change Mitigation and Adaptation, Oil Spills Clean up and Remediation, Environmental Toxicology & Health, Emissions Control and Respiratory Protection, Biogeochemistry, Earth sciences - Atmospheric sciences, Earth sciences - Climate change, Earth sciences – Limnology, Health care - Health education, Health care - Health policy, Health care - Health services research, Pollution research and control, Public health and epidemiology - Biostatistics and methodsPublic health and epidemiology - Chronic diseases, Public health and epidemiology - Environmental health, Public health and epidemiology – General, Public health and epidemiology - Global health, Public health and epidemiology - Health behavior, health promotion and society, Public health and epidemiology - Health policies, systems and management, Public health and epidemiology - Occupational health
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 23 Sep 25 |
read | read |
|
Version 2 (revision) 22 Jul 25 |
read | |
|
Version 1 17 Mar 25 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)