Keywords
Life after stroke, platform trial, evidence review, stroke outcomes, stroke rehabilitation, adaptive randomised controlled trial
In the UK, over 100,000 people have a stroke annually. Over 1.3 million live with the effects of stroke, including problems with mobility, communication, cognition, anxiety, depression and fatigue. Previous research has tested single interventions to improve stroke outcomes in separate, fixed design, parallel-group trials. Evidence generation has been slow and inefficient. Adaptive trial designs are required, to better understand multiple treatments, targeting multiple questions simultaneously. We undertook to develop the first efficient adaptive platform trial protocol, aligned with national research priorities for ‘Life After Stroke’.
We embedded PPI activities throughout the platform development and co-developed resources to maximise equality, diversity, and inclusion.
We established an inclusive multidisciplinary collaboration to inform design choices and future UK-wide platform delivery. We scrutinised existing research to identify candidate interventions and relevant outcomes; agreeing these with collaborators and PPI. We undertook detailed simulations to inform choice of platform design (adaptive elements, allocation, numbers of interventions, decision criteria for dropping/adding arms, interim analyses timing/frequency; intermediate outcomes choice). We explored barriers to trial and intervention participation through in-person and virtual meetings. To facilitate rapid trial set-up, we engaged with stroke research leaders and data providers; reviewed platform randomisation requirements; and widely disseminated our learning.
We developed an efficient, adaptive trial protocol, which is feasible, inclusive and acceptable to stroke survivors and services and submitted a funding application for a platform trial testing at least five pre-determined non-pharmacological interventions for post-stroke emotional difficulties, the top research priority for ‘Life After Stroke’.
The complex and inclusive platform trial design has only been possible through UK-wide multidisciplinary collaboration with stroke researchers, trialists, clinicians, methodologists, third sector, and patient and public contributors. Such a trial would be a step-change in trial design, reducing research waste and accelerating evidence generation to inform improved stroke service provision world-wide.
To develop a new research approach to test out therapies to help people with life after stroke.
A stroke happens when the blood supply to part of the brain is cut-off. Although hospital care has improved, over a million survivors of stroke live with long-term effects such as impaired communication, reduced mobility, and mental health difficulties.
Previous research has tested approaches to address these problems one at a time. This takes a long time and does not consider the complex and varying needs of stroke survivors. We are reporting the work undertaken to design research known as a platform trial, which tests multiple ways of helping people in a single efficient study.
We brought together a team (collaboration) of stroke survivors, carers, healthcare staff and stroke researchers to help us design the first platform trial to improve lives after stroke. This collaboration met regularly and together we:
Explored different study designs to decide which would work best.
Discussed with survivors of stroke what was important to them.
Agreed on how to select therapies for the study and reviewed research papers to find promising options.
Investigated what is relevant and important when measuring the impact of therapies to improve life after stroke.
Discussed why stroke survivors might find it hard to take part and how we can make it easier for them.
Applied for NIHR funding for the platform trial and prepared to set up the research quickly if the application is successful.
Our patient and public involvement and engagement (PPIE) groups met regularly to ensure the research would meet the needs of stroke survivors.
We raised awareness of our planned platform trial in life after stroke at events including the UK Stroke Forum and European Life After Stroke Forum.
Life after stroke, platform trial, evidence review, stroke outcomes, stroke rehabilitation, adaptive randomised controlled trial
A stroke is a serious life-threatening medical condition occurring when the blood supply to part of the brain is cut off. Stroke survivors are often left with long term problems caused by the brain injury; stroke is the leading cause of complex adult disability1,2. Globally, one in four people will have a stroke in their lifetime3. In the UK, over 100,000 people have a stroke every year - one every five minutes and there are now over 1.3 million stroke survivors4. Stroke costs the NHS and social care £8.6 billion per year5. Stroke incidence differs by ethnicity, with an increase in the black population6,7. Incidence rates are rising across all age groups. Older people are at highest risk and with our population ageing, there is a predicted incident increase of 60% by 2035, with numbers of stroke survivors predicted to more than double8. With increased burden of care, societal costs are expected to almost treble8 from current estimates of £25.6 billion per year.
Research has improved in-hospital care (e.g. new emergency treatments, specialist stroke units), leading to better survival rates9. Coupled with rising incidence rates, many more people now live with post-stroke disability10. Around one-third of stroke survivors have a moderate or severe disability; three-quarters report limb weakness; one-third have impaired communication; two-thirds may experience visual problems; more than half experience anxiety or depression; fatigue affects at least half; a range of cognitive problems are reported; and other ‘overlooked’ effects (neglect, sensory loss) severely affect quality of life4.
Previous pragmatic trials have addressed these problems with single interventions to broad populations, often failing to find evidence of effectiveness, reflecting that stroke survivor needs are complex and multifaceted. There has been an increase in the development of promising interventions11. We now need to evaluate these using more efficient methods to provide the evidence to improve post-acute care outcomes for all stroke survivors i.e. ‘life after stroke’, with awareness of the demographic and socioeconomic disparities both in the burden of stroke and provision of healthcare12.
The stroke rehabilitation and long-term care priorities set out by the 2021 James Lind Alliance (JLA) Priority Setting Partnership, highlighted the need for research to improve care for ‘mental and emotional’ (psychological) problems after stroke, stroke services in the community and understand the long-term impacts of stroke on everyday life. We aimed to develop a platform trial to provide evidence relevant to the top 10 JLA priorities and contribute to a better understanding of effective post-hospital stroke rehabilitation models, a NHS Long term plan1 milestone, informing the National Stroke Programme13,14.
‘Life after stroke’ interventions have previously been evaluated in separate conventional parallel group two-arm randomised controlled trials (RCTs), after establishing feasibility and proof of concept. This is slow, inefficient and does not allow simultaneous evaluation of multiple (or combinations of) interventions, nor is it flexible to respond to new interventions becoming ready for testing during trial implementation or allow futile treatments to be discarded before trial end11,15. Global stroke recovery research leaders have called for efficient, but more complex adaptive designs, enabling better understanding of multiple treatments, targeting multiple questions simultaneously16.
Stroke survivors have multiple challenges: addressing each in isolation is inefficient and prevents answering questions of real relevance to survivors. An efficient adaptive platform trial (APT)17 design, with common platform infrastructure, efficient use of control arm(s) and ability to streamline the launch of new trial interventions, would speed up evaluation and answer multiple questions. It could adopt a broader, inclusive ‘person-focus’, so pertinent to a population with varying needs, rather than the traditional, narrow ‘intervention-focus’.
Thus, we planned to design a platform trial to test multiple interventions, aligned to stroke survivor needs, to increase understanding of which interventions targeting a particular issue, such as reducing post-stroke psychological problems are more effective. Such a platform trial would also facilitate understanding of how the interventions work in the presence of co-existing post-stroke problems, such as physical disability, fatigue, cognition or communication, which may present barriers to intervention adherence and reduce intervention benefits.
Despite recent growth in the number of platform trials, very few are designed to test interventions other than drugs or vaccines18. Therefore, we needed to work through the design implications for complex intervention evaluation and ensure the proposed platform methodology addressed key issues such as clustering, compliance / adherence, flexibility in intervention delivery, mechanisms of action. As specified in MRC-NIHR guidance, we needed to consider how to efficiently embed process evaluations within platforms to understand for whom, how and why the interventions work and in which context. Few of these issues are addressed in the literature, so the Acceleration Award was essential to allow time to consider the best methodological approach for the platform.
The platform collaborative brought together during the Acceleration Award determined how best to design an efficient and feasible platform to address clinically relevant questions aligned to JLA priorities; The Acceleration Award also provided an opportunity to raise awareness and ensure understanding of this innovative trial methodology with the PPI, clinical and research stroke communities to facilitate co-design of these complex trials; to with other researchers designing or implementing platform trials testing complex interventions, to share knowledge and address challenges via collaboration, optimising the methodological robustness of the platform funding application to NIHR HTA.
Develop a UK platform trial to evaluate multiple complex interventions to improve outcomes for the diverse population living with stroke: the Life After Stroke Platform (LEAP)
a. Extend relationships with key stakeholders (including those with lived experience of stroke) to underpin the existing extensive multidisciplinary collaboration to support UK-wide platform delivery.
b. Agree a process for identifying and prioritising potential interventions for ongoing inclusion in the platform and identify and describe interventions and comparator to be evaluated in the platform.
c. Determine appropriate trial and economic evaluation methodology to evaluate the selected multiple complex interventions within the platform, addressing issues, such as factorial vs multi-arm designs, adaptation elements, when and how to add or drop arms, outcomes, sample size, decision analytic model structure, process evaluation.
d. Explore patient-level and service-level barriers to participation in proposed platform interventions, including health inequalities in underserved populations.
e. Undertake key tasks to facilitate rapid platform trial set-up.
f. Disseminate learning from Acceleration Award to research and stroke communities.
g. Develop detailed trial protocol capable of implementation across participating sites, aligned with service provision in health, social and/or third sector care settings and submit NIHR funding application for the national platform trial.
There were several key foundation stones for our work:
1) To build on the applicant team’s experience and knowledge relating to stroke rehabilitation research and platform trial design;
2) To develop collaborative relationships with experts in these fields;
3) For all work undertaken to be underpinned by advice, guidance and insights from PPIE representatives;
4) Undertake work to optimise engagement with underserved groups to ensure that trial design is responsive to the needs of all potential participants;
5) To be mindful at all times of the research priorities set out by the 2021 James Lind Alliance (JLA) Priority Setting Partnership.
Considerable work was undertaken in the 12 months of the Acceleration Award which culminated in the submission of a NIHR-HTA Stage 2 funding application for the Platform trial in March 2024. The main work packages addressing key objectives are described below.
The applicant team and named collaborators on the Acceleration Award included experienced stroke rehabilitation researchers from across the UK. However, buy-in from across the wider stroke research community was crucial to ensure that all the knowledge, skills and experience from all sectors was captured to develop and successfully deliver the platform trial. A multi-pronged approach was adopted which included:
a) Convening a series of meetings with senior colleagues in the Stroke Association, the largest third sector organisation that supports survivors of stroke, to present the concept of a platform trial. Participants included leaders of their research, social support and patient engagement workstreams.
b) Regular meetings were held between the applicant team and collaborators: key design uncertainties were discussed, and any knowledge gaps identified. Colleagues with relevant expertise were identified and invited to join the group. This process became more iterative as the interventions were identified for the platform trial, and intervention-specific roundtables were convened including stroke survivors, intervention developers, methodologists (statistical and trial management), and clinical leads to develop intervention protocols and ensure cohesion with the platform protocol.
c) Convening a two-day Stakeholder meeting with the applicant team and collaborators, including third sector organisations, stroke care professionals, researchers and methodologists. The meeting’s purpose was to formally establish the LEAP consortium and discuss and agree key aspects of the Platform design and delivery. It independently facilitated and incorporated an informal networking evening meal to enable relationship building.
The agenda covered:
• Endorsement of the methodology used to identify candidate interventions, including searches and screening.
• The process for identifying, prioritising and including interventions in the platform, and the range of post-stroke difficulties to be targeted by platform interventions (further details on page 7)
• The target patient population (ensuring the platform was inclusive of all stroke survivors, including under-served groups) and the settings (rehabilitation / community-based / up to 1-year post-stroke or beyond) (further details on page 12)
• Appropriate, feasible evaluation designs for the platform (e.g. group sequential/ Multi-arm, Multi-stage (MAMS) / factorial / basket) (further details on page 9)
• Important measures and drivers for the choice of intermediate and definitive (primary) outcomes (further details on page 9)
• Barriers to intervention uptake / implementation and enablers of inclusivity (further details on page 12 below)
• Establishing a single stakeholder agreed Decision Analytic Model framework capable of assessing all treatment costs and QALYs across the Platform.
d) Gaining methodological advice and guidance from national and international experts in the field of platform trials, process evaluations, health economics, data science (e.g. the HDR-UK BHF Data Science Centre and its recently established Stroke Catalyst) and stroke care and research (e.g. NIHR CRN Stroke Speciality Lead and Regional Leads, Scottish Stroke Research Network).
e) Convening a Clinical Reference Group, which included members of multiple disciplinary teams from across the UK with experience in delivery stroke services. Emerging ideas were discussed with the group who provided a sounding board for the development and practical implementation for the future Platform Trial.
Extending PPIE engagement and formation of the PPIE Group
The PPIE lead Holly Schofield and colleague Jess Johansson attended a local Different Strokes Group to discuss the work and recruit stroke survivors and carers who would be interested in joining a bespoke PPIE group for Platform trial development. In addition, collaborative team members identified stroke survivors and carers who had previously supported their research endeavours who were then also approached to join the group to ensure that the PPIE Group had a national perspective. An accessible flyer was designed with aphasia friendly text and QR code linking to an audio-visual version which was shared with stroke survivors and carers who expressed an interest to provide some brief information about the opportunity for involvement. Based on the needs and preferences of PPIE group members, a decision was made to split the group into two, an online group for people based across the country and an in-person group in Leeds for people based locally. Membership of the groups varied over the course of the award; with a total of ten stroke survivors and two carers contributing at different stages over 12 months. Five group members were involved from start to finish. A range of impacts from stroke was also represented by the groups including physical impairments, communication challenges (including aphasia), fatigue, executive function and memory and concentration. The local group had a stroke survivor from a South Asian background and both men and women were well represented in both groups. An informal induction to involvement was carried out both over the phone and at initial meetings to learn about any support needs, to manage expectations about involvement, and to provide an overview of the Acceleration Award, including PPIE aims.
PPIE Activities
Thereafter key tasks undertaken by the PPI group through regular meetings and one-to-one discussions were:
Identification of key priorities to be addressed by interventions
Review of the shortlisted interventions and active involvement in the intervention development groups
Review and recommendations regarding outcome measures.
Consideration of recruitment strategies to optimise recruitment.
Discussion and recommendations regarding information provision relating to platform trials
Recommendations regarding additional support stroke survivors may require to optimise engagement in the trial.
Review of activities and development of guidance for ensuring PPIE activities are accessible and inclusive to all stroke survivors including those with complex needs.
We intended to use an Overview of Cochrane Reviews Non-pharmacological interventions for longer-term stroke survivors or their carers (led by co-lead AFo)19, to summarise effective interventions addressing post-stroke challenges aligned with JLA priorities. The Overview of Cochrane Reviews is ongoing and does not capture emerging individual trial evaluations, we therefore undertook our own extensive systematic reviews – led by AFo and JG to search for existing candidate interventions. The outputs from the Cochrane Overview provided a useful reference point to provide overall context for life after stroke interventions and outcomes used in their evaluation.
Detailed and comprehensive searches were undertaken in March 2023 to identify candidate interventions. We firstly, sought to identify interventions that had evidence of feasibility and efficacy but had not been evaluated in a Phase III trial in the UK setting. We therefore undertook searches of key databases from 2015 onwards for reported RCT/Phase III trials undertaken outside the UK (as they could be evaluated in the context of the UK stroke service) and for pilot/feasibility studies undertaken within the UK. Following validation checks, the review focused on those studies identified through the Cochrane Stroke Group Doris database (https://www.askdoris.org/openversion/main.asp?opt=2709)20. This was an established database for ongoing and completed stroke studies. Due to the closure of the Cochrane Stroke Group, additional searches were undertaken to identify any relevant studies reported after the last update of the Doris database. In addition, national and international stroke researchers were contacted to check awareness of other ongoing or recently completed studies. Following initial scrutiny of identified papers (definitive RCT (non-UK) OR a UK pilot RCT (any size), in life after stroke, not using expensive equipment or knowledge prohibiting feasibility) members of the research team and collaborative undertook full text review of trials, using purposely designed eligibility criteria (See Figure 1 and Figure 2)
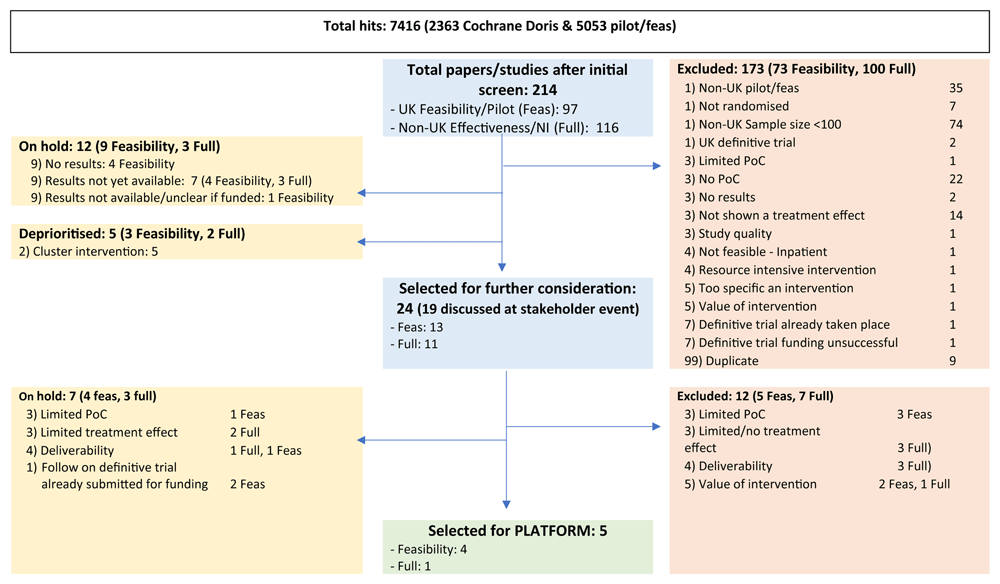
For the definitive RCTs it was ascertained whether the intervention was already the subject of a definitive trial evaluation in the UK, if not the following was considered.
Is the treatment effect:
Plausible, convincing, important to patients and clinicians?
Maintained long-term, or just post-intervention?
Only for primary outcome or wider across multiple outcomes?
For the whole sample or just a sub-group?
Identified UK feasibility and pilot studies were also reviewed to identify the most promising interventions using the following criteria:
Is there proof of concept to show this intervention is ready for trial testing?
Are there obvious ‘red flags’ e.g. poor recruitment, Intervention delivery or follow up?
Identified interventions were presented and discussed at the Stakeholder meeting and subsequent collaboration meetings and intervention roundtables (details below). Each intervention was reviewed in small table discussions through the lens of specific questions. Attending stakeholders were also asked for information on any ongoing feasibility/pilot work which although completed had not been published.
As the promising interventions to include in the Platform trial emerged, meetings were held between July and October with members of the original research team for each candidate intervention. The feedback on their original trial; potential challenges and views on including the intervention in a platform trial were sought. Some interventions were excluded at that stage if it emerged following further scrutiny that the criteria above were not fulfilled. The remaining interventions were discussed with the PPIE group prior to final selection of interventions to be evaluated in the platform trial.
Additional searching of the literature was undertaken concurrently, of the shortlisted candidate interventions, focused on the content and topic, to ensure no overlapping similar studies were being undertaken and inform all design considerations (for example, relating to the process evaluation).
The proposed interventions were also discussed with the Clinical Reference Group to assess acceptability and relevance to survivors of stroke and feasibility of and proposals for delivery in the busy NHS clinical setting.
Subsequently intervention roundtables were organised to ensure all relevant details of the intervention and original evaluation were captured.
Refinement of interventions
The Intervention roundtables attendees included the core LEAP team, key LEAP collaborators, including members of the PPIE group, and the researchers responsible for developing the candidate interventions. Discussions focussed on the interventions and the pilot/feasibility study or RCTs in which they featured, paying particular attention to elements of TiDIER, methods for implementation, outcome measures, feasibility of delivery in the NHS and the LEAP platform and adjustments that could be made to increase accessibility of the interventions. The Roundtable meetings informed the design and proposed protocol of the platform trial.
The TIDieR framework was used to describe the content, context and implementation of the candidate intervention as evaluated in the original trial. Particular attention was paid to the eligibility criteria, intervention setting and location, intervention duration and format, and timing of intervention delivery to evaluate the comparability of interventions and to inform the overall platform focus, eligibility and design. During the intervention roundtables, feedback from all parties was gained for the acceptability, feasibility and relevance of the intervention.
The applicant team for the Platform trial was re-configured to include the original intervention developers, regional leads, and colleagues with expert knowledge in the focus of the interventions, as well as those with specific methodological expertise.
Statistical considerations
Informed by current good practice recommendations, the statistical team (AW-H and AJF) used the Acceleration Award to address critical uncertainties and review platform trial design choices. This included consideration of the implications for complex intervention trials for the design of the platform and aimed to address the following design features:
a) Multi-arm vs factorial designs for testing multiple interventions, given that stroke survivors may be eligible for multiple interventions, and whether specific components of interventions should be evaluated on an individual or whole systems level
b) Maximum numbers of interventions within the platform at any one time
c) Decision criteria to assess whether interventions are ready for evaluation within the platform and at any interim analysis (e.g. dropping arms for efficacy or futility, or including new arms), particularly as early phase trials of complex interventions tend to focus on assessing feasibility and conducting pilot work, rather than assessing potential efficacy
d) Timing and frequency of interim analyses
e) Approach to halting or continuing recruitment during interim analyses
f) Choice of intermediate adaptation and definitive outcomes
g) Allocation ratio between comparator and intervention arms
h) Clustering of participants by site, treatment provider, or other contextual factors relating to setting as common in trials of complex interventions; impact on unit of randomisation, contamination between trial arms, sample size requirements, and analytical plan considering multi-level data structures.
i) Measurement and evaluation of intervention compliance and adherence, and permitted flexibility in intervention delivery; integration within the platform to inform interim analyses and the decision to add or drop arms
j) Simulation to establish and understand how decision criteria and design parameters affect the operating characteristics (power, type I error, pair-wise & family-wise error rates) of alternative statistical designs
k) Consider practical implications of delivering statistically complex designs
Methods to address these aims included:
Building on experience within Leeds Institute of Clinical Trials Research (LICTR), LEAP methodologists sought insights from colleagues involved in the design and/or delivery of previous Platform studies, predominantly in oncology settings (CONCORDE, PLATO, FOXTROT, RADAR, FLAIR) but also other NIHR-HTA Platforms incorporating some elements of complex interventions, but in non-stroke settings (MODULATE, MIDFUT21,22). Broad platform design concepts and options were presented to the LEAP collaborators during the stakeholder event, and attendees worked in groups to consider both their preferred design, and appropriate and feasible designs given the interventions identified at that point in time. Discussion and feedback were taken on board to subsequently configure a series of LEAP intervention specific platform design options (see Figure 3) which were circulated for feedback and discussion in further collaborator and PPIE meetings before confirming trial design through consensus agreement.
We approached the issue of clustering of participants by restricting interventions eligible for inclusion in the platform to those able to be delivered individually, rather than in a cluster randomised setting. We considered the need to inflate the sample size (to maintain adequate power to detect specified treatment effects) to account for interventions delivered by healthcare professionals in which outcomes among patients treated by the same person are likely to be more similar with sample size inflation dependent on: the clustering structure, intra-cluster correlation (ICC), number of therapists (or equivalently patients per therapist), and coefficient of variation of cluster size.
We also considered the efficiency gains in baseline adjusted analysis and extracted estimates of the correlation between baseline and follow-up outcomes in a previous stroke rehabilitation trial delivered by the Academic Unit for Ageing and Stroke Research (ASR) and Leeds Clinical Trials Research Unit (TRACS)23, where there was consistency with planned outcomes. In response to decisions regarding the planned primary and intermediate outcomes, we also extracted estimates of the correlation between different outcomes, and between outcomes measured at different follow-up timepoints using the same trial.
To support evaluation of the impact of our design decisions on the sample size, design operating characteristics and analyses plan, we made use of available resources on platform methodology, attended the University of Cambridge MRC Biostatistics Unit course on Adaptive Methods in Clinical Research and sought expert advice and collaboration from the course lead. This additional collaboration enabled extensive bespoke simulations and analytical derivations of the proposed platform design.
Health economic considerations
Economic evaluation often uses mathematical models to compare the expected health benefits and costs across counterfactual treatments. For any given piecewise comparison of treatments in isolated trials, different model structures introduce additional and unnecessary ‘structural’ uncertainty which is difficult to evaluate. Platform trials permit construction of a reference case model in which all included treatments can be evaluated across the relevant populations. Such reference case models are argued to minimise modellers bias, more accurately capture the progression of the condition, lead to more accurate estimates of costs and health outcomes, and thus potentially providing earlier access to new cost-effective health technologies24. Additional efficiencies can be gained by pre-specifying model parameters which will be generated within the trial thus simplifying the process of evidence generation and minimising the potential for important evidence gaps.
Access to stakeholders permits generation of a model more likely to capture the important drivers of costs and benefits and collaboration with the statistical team ensures appropriate data generation and consistent approaches to issues such as missing data.
A further consideration is that not all treatments considered within the platform may be relevant for all patients. Thus, although the methods for incremental comparisons across multiple treatments are well established, the health economic modelling will need to accommodate patient heterogeneity formally in the modelling.
A further health economic concept which was discussed during stakeholder meetings was the role of Value of Information (VoI) techniques that can help inform the decision on which treatments to take forward. VoI analyses are common in economic evaluation and identify whether and what future research is worthwhile. However, they often occur at the end of research programmes, and it is unclear whether they have any real impact on future research direction. Embedding such analysis within a platform trial has the potential to maximise the impact of this approach.
Selection of outcome measures
Further work was undertaken to identify the most appropriate outcome measures to evaluate across the platform and for selected interventions (led by AWH & AFo). We did do so by 1) reviewing Core Outcomes Sets for evaluating post-stroke interventions, 2) focussed discussion of important measures and drivers for the choice of intermediate and definitive (primary) outcomes during our 2-day stakeholder meeting, and 3) using Intervention roundtables to establish how proposed outcome measures fit with specific interventions, their mechanism of action and logic model. Statistical and trial design implications were considered at each stage including the strength of evidence for the reliability and validity of outcomes, minimal clinically important difference, timing and correlation of intermediate and definitive outcomes, and sample size.
Given the breadth of life after stroke interventions under consideration, we expanded our review of outcomes to include previous and ongoing stroke rehabilitation trials according to multiple Cochrane reviews informing the top 10 JLA Priorities and collaborator involvement, together with the those identified in the previous and ongoing Overview of Cochrane Reviews19. We extracted and compiled all measures, broadly grouping these into outcome domains providing a reference set of outcomes. Prioritisation and ranking exercises were subsequently undertaken with the stakeholder and PPIE groups to identify the outcome domains of most relevance to the needs of survivors of stroke, and relevant to each intervention, followed by detailed review of specific outcome measures. Consideration was given to the method, timing and frequency of outcome measurement, research efficiency and minimising participant burden, and whether collaborators, patients and stakeholders would trust in the findings of selected outcome measures when making decisions to drop or amend trial intervention arms.
Process evaluation development
Process evaluations provide an invaluable understanding of how, why, for whom and in which context interventions do or do not work. A separate expert group (led by LK and Jess Johansson) was convened to review the methodology related to ongoing and previous process evaluations of complex interventions in stroke rehabilitation. Initial plans were then refined by the collaborative group. Methodology was developed to ensure the process evaluation was designed to take advantage of and optimise outcomes for a Platform trial as well as refining programme theory relating to the included interventions.
Given the novel nature of this platform trial in stroke rehabilitation, it was considered important that the PE also be used to explore factors that affect the running of the trial to help optimise the design and conduct of future platform trials in rehabilitation research.
We reviewed previous approaches within stroke trials to optimise recruitment and to sustain engagement from underserved populations. We met with the CTRU Equality, Diversity & Inclusion (EDI) Lead to discuss our approaches and examine routes for future engagement with underserved groups during later stages of the trial development (if funded). The WAterS-225 PI also provided information on their EDI informed PPIE approaches and raised some queries from the LEAP team around terminology with the WAterS-2 PPIE Research Advisory Panel which helped to inform our plans for describing interventions targeting emotional difficulties.
Scenarios for recruitment strategies to maximise reach and engagement with the trial for potential participants were developed. These were iteratively refined with the PPIE group and collaborator team and further amended when the recruitment windows for each intervention were confirmed.
Our PPIE group and Clinical Reference Group were asked to identify and offer suggestions to address this key design challenge for our proposed platform trial. We explored various trial adaptations and reviewed the proposed intervention materials to ensure that they were accessible for stroke survivors with cognitive impairment, including language difficulties.
We completed the worksheets for the INCLUDE Ethnicity framework as part of our Acceleration Award activities.
The LEAP team undertook the following activities to enable rapid platform set-up: Defining strategies to improve set-up time including consolidating learning from CTRU trial teams conducting adaptive platforms trials and through raising awareness of platform designs within the UK and stroke research and clinical communities. See Table 1 on Acceleration Award dissemination26. Utilising the LEAP team’s extensive experience with stroke trial delivery, the LEAP team was able to identify the challenges of platform implementation within UK stroke services and incorporate strategies in the planned platform trial to meet these challenges.
During the Acceleration Award, the LEAP team developed resources and strategies for providing information around complex designs and methods for potential participants, working closely with the PPIE group to establish the most effective ways of presenting this material.
Plans for staged information provision (Figure 4) specific to the LEAP platform and candidate interventions were developed in consultation with the CTRU Quality Assurance team, incorporating both ethical and regulatory requirements into the stages. The plans were presented to the PPIE group who were provided with the opportunity to comment on and refine the proposals.
The LEAP team met with the CTRU Information Systems team, who are accustomed to collaborating with statistical and data management teams to specify randomisation systems capable of meeting the requirements of complex platform designs. Together we worked through the specific requirements of the LEAP platform randomisation system, to enable an efficient set-up of the system, should the Platform trial be funded.
Pro-active engagement with the stroke lead for the NIHR Clinical Research Network (now Research Delivery Network) was initiated prior to Acceleration Award submission. The CRN (RDN) National Specialty Lead for Stroke is supportive of our work and has ensured direct engagement with the English regional stroke leads. Similar activities and response have been undertaken with the stroke research network in Scotland.
Discussions with Sentinel Stroke National Audit Programme (SSNAP) and HDR-UK BHF Data Science Centre Stroke Catalyst were initiated to understand the potential for the use of routinely collected data from electronic health records and registries/audits within the proposed Platform trial.
Identification of sites
Engagement work with specialist stroke networks was undertaken to facilitate the identification of potential LEAP sites. Methods to identify sites included engaging with these networks, such as the CRN (RDN), and via national dissemination events, such as UK Stroke Forum and the European Life After Stroke Conference. Drawing upon the extensive experience of the LEAP Acceleration team from both the ASR and CTRU in the successful set-up and delivery of stroke trials, the LEAP team were able to engage existing collaborations and research networks across NHS sites to gather interest and feedback on the proposed platform trial.
Extensive dissemination activities have been undertaken, including presentations and workshops at local, national and international stroke groups/conferences, to increase understanding of innovative trial methods and build capacity to deliver complex interventions platform trials within the stroke research and clinical communities.
The ultimate focus of all Acceleration Award work was the development of a NIHR-HTA application for proposed Platform trial, with the Stage 1 application submitted in November 2023 and the Stage 2 application submitted in March 2024, including a twenty-page detailed research plan/protocol.
Colleagues in the Stroke Association became key supporters in the development of the Platform Trial. Their Director of Research Richard Francis attended the Stakeholders meeting in Leeds and he, or his deputy Emily Griffin, attended all collaboration meetings. A meeting was held with colleagues from the Stroke Association in April 2023, including the Research Involvement lead, at which involvement of people with lived experience of stroke, and ensuring diversity in involvement and recruitment were discussed. Following this meeting, the research team were introduced to public contributors by email, one of whom joined the PPIE Group for the award.
The original team of applicants and collaborators was expanded to include colleagues with specific skills and insights (e.g. Professor Bojke, Health Economics; Dr Edwards, Primary Care Dr Jess Johansson, Process Evaluation; Dr Pavel Mozgunov, Statistics). The group was refined further to a co-applicant team and a Platform Clinical and Scientific Advisory Group. The former includes the original developers of the platform interventions and colleagues with methodological expertise (health economics, process evaluations, platform trial statistical design and analysis) and experienced stroke researchers and trialists colleagues from across the UK to provide geographical spread to aid implementation.
The Platform Clinical and Scientific Advisory Group includes experienced stroke trialists, academic/NHS clinicians and representation from the Stroke Programme at NHS England, the Sentinel Stroke National Audit, the BHF Data Science Centre Stroke Catalyst and HDR-UK, as well as methodologists for both mental health trials and platform trials. They will provide expert advice for scientific, operational, clinical or implementation challenges and review blinded information relating to trial adaptation.
A schedule of all meetings is provided in Table 2.
PPIE engagement
Six PPIE meetings were organised around study development milestones to inform decision making. We used slides, diagrams and pictures, ranking or scoring exercises, and open-ended questions to support discussion.
PPIE group members joined Intervention roundtables to review suitability of platform interventions. Discussions about what makes a difference after stroke informed the decision to focus on emotional wellbeing interventions.
Roundtable Meetings with the research team, where shortlisted interventions were discussed. We removed an upper limit on time since stroke from eligibility criteria for all but one of the interventions (which is delivered within six months of stroke onset), as PPI group members felt strongly that “stroke is for life”.
We explored which outcomes matter most to stroke survivors and group members. The group evaluated proposed measures for their accessibility and relevance, and then ranked them. Mood and Quality of Life were the highest ranked outcome domains with a preference for PHQ-9 and GAD-7 as the preferred measures for the former. There was no consensus on Quality-of-Life measures, but several group members preferred a stroke specific scale, which contributed to the decision to use the Stroke Impact Scale.
We discussed recruitment strategies and how to approach stroke survivors to take part in the trial. The PPIE group felt it was important that the trial is accessible to stroke survivors with a range of needs and from different backgrounds, necessitating multiple recruitment routes and methods. They suggested where to promote the study to enable stroke survivors not receiving community care to self-refer, e.g. support groups, faith groups, social media.
The PPIE group strongly supported a staged information provision process during recruitment. Information will be kept to the key essentials when consenting to contact, with further information provided when contacted by a researcher. The group discussed the language used to describe mental health and how to describe platform studies. These discussions informed development of participant information materials. It was agreed that information on platform studies should focus on participant randomisation to one of multiple interventions. Further information on platform study design could be optional and provided via a website or on request but should be straightforward and aided by pictures.
The PPIE group considered the support for participants required during recruitment, including resources for recruiters, and they made recommendations on guidance and training for study recruiters. This included previous experience of working with stroke survivors, training on aphasia, providing options for video calls or home visits where necessary as part of consent and data collection, providing reassurance, alternative phrasing of questions to aid understanding. We will incorporate this feedback into the development of materials for study recruiters.
Guidance on inclusive PPIE approaches for stroke survivors was co-developed, informed the platform design and was shared with other researchers e.g. poster at European Life After Stroke Conference in Dublin in March 2024. This guidance explored adjustments to PPIE activity to improve accessibility and recommends that as the effects of a stroke can vary widely from person to person, it is important that PPIE approaches are tailored to everyone’s specific needs and challenges. It provides suggestions on ways to adapt activities and ensure that stroke survivors are well supported, whilst also highlighting the need for a bespoke approach.
An initial validation search generated 13,560 hits. This became the Master search. Due to the large number of hits a provisional search was undertaken on this search using the term Phase III (in the title/abstract/key words). This produced 142 hits, and after review using the below criteria, identified three trials all of which had been captured by the Doris database (search results below):
RCT
Not undertaken in UK
Feasible to deliver in a large trial (so not requiring high level specialist knowledge or expensive equipment for example, treadmills).
Broadly tackling life after stroke (as per areas identified in JLA PSP).
Some evidence of effectiveness
The Cochrane Stroke Group Doris database plus the supplementary search to update to present produced 2,363 hits, and the initial screen produced 183 papers for full review, 151 of which were identified as RCT’s from outside the UK for further review.
The separate search undertaken for pilot or feasibility studies of post-stroke interventions from 2015 onwards produced 5,053 hits. All identified titles were screened using the pre-determined criteria, and the initial screen produced 442 papers for full review, 60 of which were identified as UK feasibility trials for further review.
Colleagues at a collaborators meeting were asked to put forward any other promising interventions that they were aware of. Recommendation was made to take guidance from the Cochrane Stroke Group regarding promising Chinese trials as colleagues had just undertaken a review of these trials. Three papers emerged from this process: Patchwood27 (then unpublished now 202427), Zhang 200428 and Lawrence et al., (then unpublished29) .
In total, 214 studies were subject to detailed review by the research and / or members of the collaborative group using the decision aid (Figure 1). A total of 24 were prioritised for discussion at the Stakeholder meeting or subsequent meetings. Provisional decisions were made about possible inclusion (or exclusion) in the Platform, after which, contact was made directly and where possible individual meetings were held with the original intervention designers to clarify any methodology and feasibility issues and whether they would consent for the intervention to be evaluated in the platform trial.
In conjunction with this detailed search, review and prioritisation work, through multidisciplinary workshops and our PPIE group, we agreed to focus the platform on emotional difficulties and their impact on social participation. The citation searches undertaken on the prioritised intervention were reviewed; relevant Cochrane Reviews, Clinical Guidelines, key papers and policy documents relevant to emotional difficulties post-stroke were considered in detail; and the on-going studies portfolio was scrutinised1,2,14,30–44.
We concluded that the evidence gaps would not be met by ongoing stroke studies nor by non-stroke mental health interventions. From discussions with authors of intervention trials and stroke survivors we selected promising, nonpharmacological, candidate technologies with the strongest evidence, including efficacy, feasibility and acceptability. Five interventions were selected for inclusion in the platform, addressing the top JLA priority of emotional (psychological) difficulties. See Figure 1 and Figure 2.
Identify and describe interventions and comparator to be evaluated in the platform
Intervention roundtables were held between July and October 2023. The meetings involved the intervention developers, PPIE group and the LEAP research team with key collaborators.
Key outcomes of the intervention roundtables included:
1. Working through intervention protocols and TIDIER checklist; See Figure 5.
2. Agreement of target participant group and eligibility criteria for each proposed intervention,
3. Decision-making on how best to implement the intervention within the relevant settings
4. Reviews of how proposed outcome measures fit with the intervention mechanism of action and logic model;
5. Opportunities to streamline delivery of the interventions within the platform infrastructure were identified and agreed for the stage 2 application. Discussions included consideration of remote delivery models for appropriate interventions;
6. Further adaptations to make the interventions more accessible were explored. This resulted in the development of a training strategy for intervention deliverers tailored around speech and language difficulties;
7. Agreement from PPIE group members that the candidate interventions addressed important needs after stroke and should be included in the platform.
Key discussion points arising from the Stakeholder meeting feedback included:
A positive response to umbrella and multi-arm trial designs, with consideration of allowing for patient choice and preference for a package of interventions vs randomisation between appropriate interventions whilst avoiding burden of interventions on patients and providers. A preference emerged for the evaluation of single interventions at a patient level as opposed to multiple interventions per participant (for instance within factorial trial designs or through participant re-randomisation).
An overarching design including interventions to help prevent post-stroke symptoms, as well as those to improve symptoms in those in need, was attractive to stakeholders. There was a steer towards interventions focused on wellbeing with the potential for impairment-specific interventions to be introduced to both the participant, and into the platform at a later time.
In regards to interim analysis and intermediate decision making, there was a strong steer towards the inclusion of all promising interventions, rather than a ‘pick the winner’ or ‘drop the loser’ design. There was agreement that interventions should only be dropped for futility, rather than simply retaining those with the highest levels of efficacy in the platform. The importance for the evaluation of adherence and intervention delivery alongside statistical/outcome criteria was highlighted. It was agreed that the Platform design should incorporate flexibility in decision-making according to prespecified criteria, with care over measures of fidelity should they be included in decision criteria.
Health economics proposed a Value of information (VoI) analyses alongside all decision criteria. Structured VoI methods identify those parameters which are both uncertain and influential in determining the cost-effectiveness argument. Further research on those specific parameters will reduce the likelihood of choosing fewer effective treatments and avoid the consequences of lost health and unnecessary costs which can be quantified using the economic model. Thus, it can be used as a tool to prioritise future research such as choosing which interventions to carry forward. Such analyses can be very technical, complicated and potentially a bit black box. Just as with economic evaluation it was recognised as having potential value in a decision-making process and that it should be explored in this case rather than adopted as a formal criteria.
Following the stakeholder event, and identification of candidate interventions, three intervention specific platform designs were proposed for feedback (Figure 3)) a single all-comers multi-arm multi-stage (MAMS) trial, open to as many stroke survivors as possible with common eligibility criteria, 2) an umbrella trial design consisting of separate trials according to trial/intervention specific eligibility criteria and 3) an overarching MAMS trial with randomisation schedule informed by intervention-specific eligibility criteria and a single overall shared control arm.
Whilst ambitious the efficiency of an overarching 2-stage MAMs design was the preferred design, providing the ability to answer multiple research questions within and across patient sub-groups.
A common concern was the concept of a common primary and set of secondary outcomes applicable to all participant sub-groups and interventions. Whilst this would maximise platform efficiency, there was concern whether a common primary outcome would be sensitive to heterogeneous interventions and sufficiently sensitive to change, particularly at interim decision criteria. As detailed in our results on outcome selection, our final trial design included two primary outcomes for the platform, to ensure appropriate and applicable evaluation of interventions according to their respective mechanisms of action. In the absence of true surrogate outcomes, and to ensure the timing of interim analyses would not become obsolete, we selected to use the same two primary outcome measures but measured at an earlier timepoint as intermediate outcomes; with measurement at 12- and 6-months post randomisation for final definitive primary outcome and intermediate outcome respectively. The decision to include two primary and intermediate outcomes introduced additional statistical complexity in terms of decision criteria, sample size and operational characteristics of the platform design. We specified decision criteria at interim analysis such that a promising signal on at least one intermediate outcome was required to proceed to the stage 2 final definitive evaluation, with interventions dropped for futility if shown to be futile on both intermediate outcomes. We similarly specified that the stage 2 final definitive effectiveness evaluation was based on observing effectiveness on at least one of the two primary outcomes. To evaluate the impact of these decision criteria on the required sample size for each arm, we incorporated estimates of the anticipated correlation between the two outcome measures, and the correlation between measurement at the 6-month intermediate and 12-month primary outcome timepoint, allowing for uncertainty according to the 95% CI around each estimate.
Further issues were the sample size and expected distribution of participants across interventions and the common control, according to eligibility criteria for each intervention and overall complexity of the design. We considered alternative approaches to randomisation in this context, including the potential for weighted randomisation to provide control and equality across the number of participants allocated to interventions. We ultimately decided on a pragmatic approach, respecting the underlying proportion of participants eligible for interventions, and thereby maintaining generalisability of findings which would arise from the trial.
We modelled the anticipated proportion of recruited participants who would be eligible for each intervention according to estimated rates of anxiety and depression35,45–47 and timing post stroke. This informed the anticipated recruitment rate to each intervention arm, at the start of the platform when all interventions would be open to recruitment, and over time as interventions each reached their recruitment target. This also informed the number and anticipated proportion of participants in the shared control arm who would be eligible for comparison to each intervention, thus informing the total anticipated sample size in the control arm as well as pairwise proportion of participants in the shared control across interventions. Through modelling the process of randomisation to interventions or control, dependent on the estimated proportions of participants eligible for each intervention, we reconsidered the timing of our interim analysis, and based this on 40% rather than 50% information to maximise the benefits to the platform from the possibility of early stopping whilst maintaining appropriate power.
Extensive simulations and analytical derivations were used to establish and understand how our decision criteria, multiple primary and intermediate outcomes, timing of interim analysis, correlation between outcomes and timepoints, partially shared control arm affected the platform operating characteristics. These included the power according to different specifications (globally, by arm and outcome), the pairwise and family-wise error rates.
As interventions were complex, we included internal pilot progression criteria to allow interventions to be discontinued for futility, feasibility, or acceptability after 12 months of recruitment and an additional 4 months of intervention delivery. At interim analysis, we specified non-binding futility decision criteria to also allow consideration of the same progression criteria, as well as a value of information economic evaluation.
Additional methodological consideration was given to the content of the control arm and contamination. We considered standardising the control group through the provision of stroke association information leaflets, containing generalised stroke information as well as more specific information around mood and physical recovery. Due to the focus on life after stroke and variable timing at which patients could enter the platform we selected a usual care control instead. Recognising the variations in usual care provision across sites, we considered the complexities and options for collecting data on usual care using routine data, rather than relying on information directly from participants themselves. This included exploration of data with the BHF Data Science Centre, Sentinel Stroke National Audit Programme (SSNAP) and NHS England (HES etc)48,49. In terms of contamination, we examined TiDIER checklists for each intervention to identify risks. We aimed to minimise contamination between multiple interventions, by organising mutually exclusive groups of staff to be trained and deliver each intervention, with none providing care to control participants. To do so, and to ensure platform and intervention deliverability, we designed the platform to minimise dependency on local healthcare structures and considered central model/s of intervention providers.
As the platform was designed to meet the needs of patients over the wide-ranging life after stroke trajectory, we considered whether to randomise participants to the timing of intervention delivery (early vs later intervention). To ensure deliverability and pragmatism, we decided against this approach. With the exception of eligibility criteria related to the timing post stroke for some interventions, the platform was designed to broadly evaluate interventions regardless of the timing post stroke, and instead incorporated an objective and appropriate approach to statistical analyses to explore the moderating effect of time-post stroke on the effectiveness of treatments in subgroup analyses (using a statistical interaction test) at final analysis. Other factors such as, recognising differing post-stroke impairments (fatigue, cognition, communication) were also included in planned subgroup analyses.
Our resulting trial design was for a multi-centre, individually randomised, community-based, researcher-blinded, adaptive Phase III Multi-Arm Multi-Stage (MAMS) platform trial design, with internal pilot, embedded economic and process evaluations and SWAT, testing interventions for post-stroke emotional difficulties compared to usual care control.
Selection of outcome measures
Our initial search for existing outcomes identified a number of Stroke symptom specific core outcome set recommendations and an international standard set of patient-centred stroke outcome measures for use in a variety of settings, however no further overarching core outcome sets were identified and unfortunately promising plans to develop and agree a core outcome set for life after stroke, the LASSO project developed as part of the COMET initiative was not funded. The recommended international standard set of patient-centred stroke outcome measures was relatively simple and included only the short form PROMIS-10 questionnaire to collect almost all patient-reported outcomes, including pain, mood, feeding, selfcare, mobility, communication, cognitive functioning, social participation, ability to return to usual activities, and health-related quality of life as single question/item responses at discharge and 90 days post discharge. There was a consensus that the single PROMIS-10 questionnaire would not be sufficiently comprehensive to rely on solely to determine the relevant outcomes for this platform, so we looked to all the 20 Cochrane reviews informing the top 10 JLA priorities, extracting detailed outcome domains and measures used and referred to within each review. Compiling measures from across these sources, with Stroke trials previously delivered through AUASR and LICTR, we identified a total 281 measures and outcomes, described using approximately 60 different outcome descriptors and aligning broadly within 9 overarching domains of 1. Quality of life, 2. Mood, 3. Activities / Extended Activities of Daily Living, 4. Dependence / Independence / Disability, 5. Physical, 6. Cognition/memory/vision, 7. Communication, 8. Carer, 9. Adverse events/ safety, and an additional group of other measures, including self-efficacy, satisfaction, health service use, drop out and intervention adherence.
During a prioritisation exercise, stakeholder attendees ranked the outcome domains in terms of their potential for inclusion as an overarching platform primary outcome. Quality of life, followed by mood and activities of daily living were rated as the top contenders (in that order) with some support for measures of independence and disability also. Group discussion and feedback highlighted the difficulty groups had with the concept of a single overarching primary outcome and a key output was to maintain sight of intervention specific mechanisms of actions to ensure appropriate outcomes are included in the platform, and to ensure the patient voice as included in all decision making regarding outcomes.
Further work collated collaborators preferred, recommended outcome measures associated with top domains, and measures to avoid. We ultimately decided upon two primary outcomes for the platform, to ensure applicable and relevant to all interventions. Decision criteria at interim analysis required a promising signal on at least one primary outcome to proceed to stage 2, and stage 2 evaluation of effectiveness was similarly based on observing effectiveness on at least one outcome. The inclusion of two primary outcomes introduced additional statistical complexity but ensured appropriate evaluation of interventions according to their respective mechanisms of action and was not reliant on a single primary outcome which would not be sufficiently sensitive across all platform interventions. The primary outcomes selected were both subscales from the Stroke Impact Scale, measuring different domains of mood and participation. We chose stroke specific rather than generic measures for the primary outcome, and included further generic measures of mood, including anxiety and depression, as secondary outcomes applicable to the whole sample.
For interim decision criteria, we selected to use the primary outcome measures (SIS mood and participation), measured at an earlier timepoint as intermediate outcomes.
We incorporated multiple methods of research data collection ensure trial data could be collected whilst minimising participant burden. We planned to supplement essential participant-reported outcome data using routine data to describe usual care, obtain data on resource use to enable full cost-effectiveness evaluation, describe participant baseline and stroke related characteristics, and enable objective outcome assessment. We included resources in our funding application to enable routine Healthcare systems data to be requested from national Audits and NHS-England (and equivalent in Scotland) to provide secondary care resource use data (hospitalisation, A&E, medications).
We planned for participant-reported data collection through postal or online questionnaires; via phone or video calls or face-to-face, according to participant preference and incorporating researcher support where indicated. To maximise retention rates, we planned to use short questionnaires, accessible materials and formats, pre-notification alerts, voucher payments, and video & newsletter updates, with reminders and increased support for non-responders, and included a SWAT to test the effect of peer-led versus researcher-led retention strategies.
Process evaluation development
The design of the mixed-methods process evaluation (PE) was informed by current MRC guidance for process evaluations50,51. The applicant team were mindful that the PE should inform interpretation of intervention outcomes of three key functions: 1) implementation, including fidelity, adherence and acceptance; 2) mechanisms of impact; 3) contexts that may influence engagement with or effectiveness of an intervention51.
The process evaluation was developed iteratively by the PE leads and the collaborative group. An overarching initial programme theory for how interventions for post-stroke emotional difficulties are expected to work will be generated by drawing on the existing programme theories available for the included interventions. Intervention documentation, published papers and protocols will be reviewed and discussed with the intervention developers to ascertain similarities across identified mechanisms, contexts and outcomes.
The multimodal process evaluation will include interviews; observations; a workshop with trial team members; document review; and integration of quantitative data on intervention delivery.
Findings will be synthesised across all datasets to specifically address intervention implementation (fidelity, adherence and acceptability) and seek evidence to refute, support or refine an overall programme theory to explain how post-stroke mood interventions work and for whom. At trial conclusion, quantitative data relating to intervention fidelity will be triangulated with the qualitative and observation data to add further richness to the PE and highlight factors for consideration in future platform trials and future intervention implementation in the NHS. Synthesis of findings from qualitative and quantitative data will contribute directly to the overall evaluation of the platform interventions.
To optimise the inclusivity and diversity of trial participants, the PE team will also undertake focus groups with recruiting staff (see page 26)
Throughout the Acceleration award and in the design of the proposed platform trial, the LEAP team has explored patient-level and service-level barriers to participation. A broad understanding of these barriers was gained through conversations with stakeholders both in our PPIE groups and our wider network of national clinical and academic colleagues.
Site-level barriers
Capacity to support research activities
The primary barrier to platform participation at the site level was identified as site staff capacity. In response to this, the LEAP trial has been designed to minimise site staff burden, to ensure site engagement and deliverability of the proposed platform. Recruitment approaches have been designed to facilitate broad and inclusive screening and approach of potential participants within stroke services, whilst reducing staff time and burden to a minimum.
The proposed platform includes a multi-modal recruitment strategy which reduces site staff burden in the screening and recruitment processes. Three main routes of approach include: 1) Site staff obtaining consent to contact while in hospital; 2) Site staff (including acute and community hospitals and GPs) mailout to potential participants from medical records; 3) participant self-referral via a website, social media or other advertisements.
In line with our PPIE-informed staged information provision, the initial approach will require minimal information to obtain consent to a contact by a LEAP researcher who can facilitate further information provision and consent.
Site staff may be asked to collect a streamlined dataset for consented participants on their diagnosis and admission information; however, this will be supplemented by routinely collected datasets, reducing the data collection time required of site researchers on the proposed platform.
Clinical site staff capacity
Candidate interventions previously evaluated in pilot and feasibility studies, alongside those from RCTs were interrogated for their deliverability in the NHS. Challenges and lessons learned from these studies informed the design of the platform intervention implementation. The deliverability of the interventions was further discussed at Intervention Roundtable meetings and with the Clinical Reference Group.
The team were able to identify ways in which the interventions could benefit from platform trial infrastructure as possibilities for standardising elements across all interventions were mapped. Clinical staffing was one such key area where the team developed a strategy. The selected interventions do not require specialist staff to deliver, and many offer the opportunity for remote and decentralised delivery models further reducing the burden on sites.
Understanding of platform processes
Challenges and barriers at a service level for both site research and clinical delivery teams include the understanding of platform trial design and governance. A workshop held at the 2023 UK Stroke Forum in Birmingham helped to identify barriers to implementation of complex intervention platform design in NHS services. Contributions from PPIE members and representatives from stroke services at the workshop highlighted the requirement for clarity regarding screening, recruitment plans and patient pathways descriptions of platform designs. Feedback and discussions from the workshop also helped to inform how the LEAP team will present governance assurances to R&D departments.
Participant-level barriers
Our PPIE group was instrumental in stressing particular barriers to engaging with research and candidate interventions during post-stroke recovery. In the design of the proposed platform, we have attempted to build in facilitators to remove some of these barriers. Identified patient-level barriers included timing of trial entry, communication, cognition challenges and accessibility.
In response to these barriers, the proposed platform includes a streamlined, multi-modal, inclusive recruitment strategy that supports stroke survivors to join the platform at a time that is right for them following their stroke.
Our planned staged information provision and step-by-step recruitment process enables potential participants to engage with the study at various points in their post-stroke journey. Using multiple routes and methods for reaching potential participants will increase the accessibility of the trial to under-served groups that may not be approached via singular strategies for recruitment.
The recruitment process will also utilise key tools, such as the Consent Support Tool, to ensure participants receive the right kind of support during recruitment and throughout their receipt of the intervention and trial follow-up. LEAP specific researchers will be trained in working with Stroke survivors with cognitive, speech and language difficulties to help support people’s participation. Getting the levels of support right at the start of the trial should increase inclusivity and support retention throughout the proposed LEAP platform.
Follow-up processes have been designed with built-in support for those participants who require it. This has also led to the design of a Study Within a Trial (SWAT) to evaluate the benefits of researcher or peer-supported follow-up data collection that will be run alongside the LEAP trial if funding is secured.
Intervention deliverers will also be provided with training to support people with speech and language difficulties helping to support participation in the trial interventions. The selected interventions are also delivered using multiple methods, both in-person and remotely, helping to reach a wide population throughout the UK.
The use of the INCLUDE Ethnicity Framework worksheets helped to identify potential barriers for certain communities to take part in the LEAP interventions and enabled the team to identify strategies to manage these barriers. These strategies were incorporated into the detailed research plan for the proposed Platform trial. We have requested funding to work in partnership with under-served community groups to make LEAP more visible in underserved communities and to translate initial trial information into priority languages, as well as using different formats of information provision (e.g. video versions).
The LEAP team also prioritised areas of high-disease burden underserved by research for the proposed Platform. This has culminated in the establishment of LEAP Hubs for research delivery in strategic geographical areas traditionally underserved by research.
The inclusivity and diversity of reach of the trial will be monitored. This will include purposively convened focus groups with recruitment staff, in which screening data will be reviewed and the barriers and facilitators to recruiting from hardly reached groups considered. Good practice will be shared, and recruitment materials amended as appropriate. This process will be repeated after 12 months of recruitment to assess impact of changes made and optimise procedures and materials further.
Database and randomisation
To ensure adequate resource and plan for future database and randomisation system, a series of meetings between statistics, trial and data management, and Information Systems teams were convened as guided by the chosen platform design, and with consideration of recommendations in Hague 202252. As described in page 19, given our Platform design included intervention-specific eligibility criteria and a single overall shared control arm, we considered different approaches to randomisation and stratification and explored the potential and limitations of existing and required randomisation systems delivered within LICTR against priority features required for the platform. To minimise complexity, we decided upon simple randomisation between eligible interventions and the shared control with equal allocation. Whilst simple, such a system would be implicitly stratified according to the criteria dictating eligibility to intervention and so would consist of a series of separate yet linked randomisation systems. As arms would recruit at different rates, according to differing eligibility rates, further stratification using minimisation would be problematic due to the bias towards arms recruiting at a slower rate, and the use of stratified lists would escalate rapidly given the different combinations of interventions participants would be eligible to be randomised to. The need to ensure a dynamic randomisation system and scalable databases, to adapt to the closure or addition of new arms, and, or changes in criteria dictating the arms between which participants could be randomised were considered.
Patient information
Staged provision of participant information will be carefully aligned to the planned recruitment pathway for the proposed platform trial; each stage will respond sensitively by providing information that helps participants to make decisions for themselves in an accessible and supported way at each stage of the process. The additional time taken during the Acceleration Award to map this pathway and associated information provision represents significant time-saving during platform set-up.
The approach was ratified by the PPIE group, and this will enable rapid development of LEAP specific participant information documents and supportive processes. See Figure 4.
NIHR CRN (RDN) & clinical engagement & site identification
The LEAP team, with the support of the CRN Clinical Lead were able to successfully bring the potential platform forward to national discussions with interested stakeholders. We presented preliminary plans for the proposed Platform trial at the online NIHR CRN National Stroke Specialty Group Meeting in June 2023 and at the in-person meeting in London in October 2023. The team also presented at a Scotland-wide stroke research network seminar where stakeholders representing clinical services attended. The Stroke network meetings helped to generate enthusiasm for the proposed LEAP Platform trial, and the team subsequently requested Expressions of interest from stroke services throughout England and Scotland. Presentation of LEAP’s preliminary plans for the platform provided an opportunity to incorporate the feedback from the CRN (RDN), clinical services and wider stakeholders and refine messaging.
Meetings held with allied professionals and experienced therapy PIs at stroke research sites as part of the convened Clinical Reference Group were integral to highlighting key areas that needed refinement in platform description and identified both challenges and opportunities for the delivery of interventions within stroke services nationally.
Dissemination activities at UK Stroke Forum (UKSF) in December 2023 provided the opportunity to network further with Stroke service staff including, research staff, R&D representatives and Allied Health Professionals.
As part of the development of the proposed LEAP Platform trial, the team identified areas of geographical importance for the trial (those areas previously underserved by research) and grouped as research delivery hubs. Once the proposed geographic areas for the LEAP Hubs were established, identified Hub Leads (designated platform application co-applicants) were encouraged to reach out to stroke services within their areas and obtain initial expressions of interest in the study.
Expressions of interest gathered from the network meetings, UKSF and via Hub Leads were collated, resulting in a list of over 50 interested sites that can be updated as plans for the LEAP Platform trial progress. Several of the interested sites have a long history of collaboration in stroke research with the LEAP team, providing opportunities to build on established relationships for successful trial delivery. Early engagement and dissemination activities through the LEAP Acceleration Award and proposed platform trial have enabled rapid set-up of the trial, if funding is secured.
The use of routine data to support the proposed platform trial was explored. through our discussions with the Stroke Data Catalyst from the HDR-UK BHF Data Science Centre and the team investigated using NHS England datasets. Although the LEAP application for the NHS England recruitment service was not accepted, the LEAP team has built in use of routine datasets in the proposed platform to streamline data collection.
Our extensive dissemination work is summarised in Table 1
We submitted our proposal (on 29/11/2023 for Stage 1 and on 21/03/2024 for Stage 2) for a multi-centre, individually randomised, community-based, researcher-blinded, adaptive Phase III 2-stage Multi-Arm Multi-Stage (MAMS) platform trial, with internal pilot and embedded economic and process evaluations, to evaluate at least five post-hospital rehabilitation and longer-term care interventions to improve post-stroke emotional difficulties .
Stroke is the fourth largest single cause of death in the UK, causing around 38,000 deaths each year1, and is the leading cause of complex adult disability2. There are now over 1.3 million stroke survivors in the UK4. There are increasing numbers of stroke survivors in the UK associated with a commensurate increase in costs.
Despite improvement in hyperacute and acute care many people still live with the consequences of stroke. Around one-third of long-term stroke survivors have a moderate or severe disability; one-third a mild disability; more than half experience anxiety or depression. Despite several previous pragmatic trials, effective interventions have not been identified to address these needs.
A more efficient adaptive platform design will speed up evaluation, answer multiple questions within a single trial, and adopt a broader ‘disease-focus’ rather than a narrow ‘intervention focus’, particularly relevant to stroke survivors with varying combinations of needs. Our work was driven by the need to address the stroke rehabilitation and long-term care priorities set out by the 2021 James Lind Alliance Priority Setting Partnership (JLA PSP).
Stroke rehabilitation research is challenging due to the complexity of the interventions, the range of service context and the heterogeneity of the participant group. This Acceleration Award allowed us to explore all facets of these challenges, ensure that the patient voice was central to our work and address the design issues of a platform trial.
We sought to be rigorous and inclusive throughout. Key to the successful implementation of this award was the establishment of strong and collegiate collaborative and PPIE Groups, both of whom contributed substantially to this large one-year programme of work.
We were fortunate to receive extensive help and guidance from UK Policy leads, third section organisations, stroke experts, people with lived experience of stroke, platform trial methodologists and researchers from across the UK.
The PPIE Group was purposely developed to ensure a diversity of views with members from different ethnicities and with a range of post-stroke impairments. Different communication platforms and venues were utilised to optimise engagement. In addition to contribution to the proposed platform trial, the recommendations generated provide a template for ours and others future work. The extensive programme of dissemination activities enabled us to expand our reach, gain concurrent feedback as plans developed and keep the stroke community (stroke survivors, their carers and researchers) aware of our emerging work.
We have summarised in the report the detailed methodological work undertaken. We felt it crucial to be systematic in the review of interventions to be evaluated in the proposed Platform, the subsequent work was meticulous and evidence based. Regular meetings of the collaborative and research team enabled sense check of emerging work at every stage and ensured that the strongest interventions emerged from our procedures, mindful to ensure that this platform was founded on open and fair process. Further refinement work was undertaken with the original trial teams to ensure all previous learning is taken forward into the Platform trial. The PPIE groups input was crucial in our ultimate focus on post-stroke emotional difficulties, an area of long-standing unmet need in post stroke care.
Trial design options were reviewed by experts in the field, options summarised for consultation and the final design reached by consensus. Whilst ambitious, the efficiency of an overarching 2-stage MAMs design was the preferred design, providing the ability to answer multiple research questions within and across patient sub-groups.
Outcome assessments were chosen by a similar robust and rigour methods which included review of previous work, expert consensus and consideration by PPIE colleagues. Health economics and process evaluation experts have joined the team. All were mindful of the opportunity the Platform provides of extending methods and optimising the learning when several interventions (rather than just one) are under evaluation. The process evaluation and health economic methods were therefore developed accordingly.
The development work undertaken will facilitate the efficient set-up of this platform trial, if successful, and other future studies in stroke rehabilitation. At the patient level this included detailed planning of recruitment methods that will enable the platform to truly encompass ‘life after stroke’, so no time limit post- stroke for recruitment will be specified. As important is the need to ensure inclusivity of hardly reached groups. Consideration of provision of general lay friendly information and trial specific information were all given careful thought and drafts co-produced by the PPIE group and researchers to optimise trial reach.
The applicant team have extensive experience of multi-centre trials in stroke rehabilitation. Early conversations were therefore undertaken with the CRN Stroke Specialty Lead, Regional Stroke Leads and other national opinion formers. Expression of Interest in trial implementation was sought to pave the way for rapid set-up if funding is awarded.
Similarly, reviews of the information system and data management processes required to implement the complex platform randomisation system will facilitate a rapid platform set, if funding is awarded.
In summary, our detailed, systematic and rigorous Acceleration Award work led to the development of an ambitious but feasible platform trial protocol.
The Acceleration Award was essential to undertake the detailed work required to design the first platform trial in stroke rehabilitation and long-term care. The complex and inclusive platform trial design has only been possible through UK-wide multidisciplinary collaboration with stroke researchers, trialists, clinicians, methodologists, third sector, and patient and public contributors. An efficient, adaptive, platform trial to address longer-term outcomes after stroke would be a step-change in trial design, reducing research waste and accelerating evidence generation to inform improved service provision world-wide.
No data are associated with this article.
The short form version of the GRIPP2 reporting checklist was used to aid the write up of PPIE activities conducted as part of this award. GRIPP2 is a guideline for the reporting of PPIE developed with community consensus53.
Figshare: Supplementary File 1_GRIPP Short Form.docx, https://doi.org/10.6084/m9.figshare.27117121.v154.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We grateful for the considerable input to the development of the Platform trial protocol from our LEAP collaborative, which includes our extensive PPIE group.
On behalf of the LEAP collaborative (in alphabetical order)
Jennifer Airlie~, Satu Baylan, Rebecca Bestwick~, Louisa Burton~, Bethan Copsey~, Tom Crocker~, Yvonne Chun#, Avril Drummond* ~, Duncan Edwards#, Jon Evans#, Stephanie Evans~, Richard Francis~, Emily Griffin, Alice Hankin, Jessica Johansson# ~, Maggie Lawrence#, Harry McNaughton#, Jonathan Mant* ~, Gillian Mead* ~, Pavel Mozgunov#, Rebecca Palmer* # ~, Emma Patchwood#, Christopher Price ~, Kate Radford* # ~, Terry Quinn, Lisa Shaw# ~, Cath Sackley*, Shirley Thomas#, Faye Wray ~
Including LEAP PPIE: Margaret Cheng# (stroke survivor & public co-applicant for platform trial funding application), Tim Myers (stroke survivor & public collaborator), Judith Thomas (former carer, family member & public collaborator), Devender Jandu (stroke survivor & public collaborator), Stuart Sleight (stroke survivor), Chris McKevitt (stroke survivor), Martin Hings (stroke survivor) Joanne Hings (family member and carer), John Murray (stroke survivor), Niamh Malone (stroke survivor)
* Named collaborators on NIHR-HTA Platform Acceleration Award Application
# Co-applicants on NIHR-HTA Platform Trial Application
~ Attended 2-day Stakeholder meeting
We are also grateful for valuable discussions with the following: Martin Dennis, William Whiteley, Martin James, Matthew Sydes, Rustam Al-Shani Salman, Maeva May, Richard Emsley.
Thank you to our Clinical Reference Group for their contributions throughout the Acceleration Award: Aoife Bennett, Kirstin Guy, Anne Eisenthal, Alwyn Sproul, Lauren Crutchley.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurology, neurosurgery, neuroscience, stroke, stem cells, extracellular versicles, traumatic brain injury.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 17 Mar 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)