Keywords
Liver disease, research partnership, health inequalities, underserved communities
Liver disease prevalence has increased dramatically in the UK over recent decades. It disproportionately affects people from lower socioeconomic backgrounds and is a major cause of mortality in working age people. There are significant geographic variations in liver disease and related mortality. Unfortunately, liver disease research has not been centred in areas with highest disease. To address this, we developed a regional research partnership in North West England, an area with some of the highest rates of disease in the country and historically low research activity.
Develop a research network growing out of the expertise developed in Manchester to Greater Manchester and Lancashire. Use national expertise in data access, and curation and decision analytics and methodology, including health economics and decision-analytic modelling, to develop an ambitious and feasible research plan. Establish links with local community organisations in underserved areas. Development of fundable and implementable research proposal to answer an unmet need in liver disease.
A multi-stakeholder network of experts was developed to identify evidence gaps highlighted by healthcare workers in areas of high disease burden and develop methodologically robust research propositions to address them. Structured stakeholder meetings helped to understand the clinical unmet needs in caring for people with liver disease, focusing on areas of high disease burden and groups traditionally underserved. These questions were developed into research proposals and presented to all stakeholders to identify proposals with greatest importance and deliverable potential.
Working alongside a breadth of stakeholders and methodological support this study developed detailed plans for four high-priority projects, establishing a shared understanding on the most critical research questions, targeted behaviours, and primary outcomes. It identified suitable methodologies to address these questions and to guide the development of effective interventions and research questions.
Through detailed discussions to address key evidence gaps in liver disease care our network collaboratively developed research proposals for national funding.
One in four people in the UK have risk factors that could lead to their liver not working. A proportion of people develop scarring within the liver, and this is usually not identified until the liver stops doing its job and starts to fail. It is one of the UK’s largest health emergencies and is a particular problem in the Northwest of England, which has some of the highest rates of liver disease in the UK.
We used knowledge of many different individuals and groups who are affected by liver disease to devise projects that could help answer important questions to improve care for people living with liver disease.
We used experiences of community leaders and people from groups at risk of developing liver disease in areas across the Northwest. We worked in partnership with a network of national experts who have experience of working out the economic impact of liver disease in the NHS. These experts formed part of the project network, alongside public, health infrastructure and clinical representatives. Meetings were held to look at how best to evaluate the economic impact of new clinical research. We also held a workshop halfway through the project to bring together the expertise of public contributors, community and health infrastructure organisations, clinicians, local authorities and researchers to discuss how best to establish areas as most in need of intervention. This workshop allowed us to devise our research question and understand evidence needed to answer it, in preparation for funding.
We developed a partnership to enable the sharing of expertise across underserved communities in the Northwest of England and make detailed plans for new, important research.
Liver disease, research partnership, health inequalities, underserved communities
The global burden of liver disease continues to increase with incidence of chronic liver disease increasing over four-fold in the UK over the last 40 years1,2. One of the most pressing challenges in addressing liver disease is that it is often diagnosed at an advanced stage, when treatment options are limited, and disease regression is no longer possible. Evidence suggests that if liver disease is identified early, up to 90% of cases are potentially reversible, making early detection a global healthcare priority2. However, methods to identify people with risk of advanced liver disease remains a challenge with no unified approach3. Moreover, optimal lifestyle preventative strategies to prevent progressive disease remain undefined.
Liver disease disproportionately affects certain regions and communities, particularly those facing socioeconomic deprivation, high rates of multimorbidity, obesity, and alcohol use (Figure 1). Despite the significant burden of liver disease in these populations, research efforts and resources are often misaligned, failing to reflect the needs of these key communities. Consequently, research outcomes may not be fully applicable to the populations most at risk, limiting the broader impact of findings.
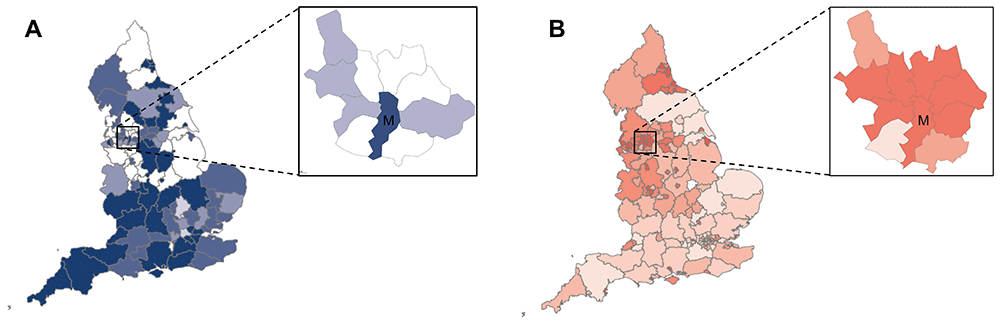
A. Heatmap of NIHR research partnership by region in England. Inset-NIHR research in the proposed partnership area. B. Heatmap of mortality in under 75 years olds from liver disease by region in England. Inset-Under 75 mortalities from liver disease in partnership area. Darker colour represents higher mortality. Manchester region marked with ‘M’ in both inset images.
To address these disparities, it is essential to engage a diverse representation of communities impacted by liver disease in research activities. This approach will ensure that research outputs are more relevant, inclusive and reflective of the diverse needs of affected populations. Our objective is to build strong research partnerships in a region with high rates of liver disease but historically low levels of research participation. By fostering these partnerships, we aim to strengthen research infrastructure, promote inclusive community engagement, and generate actionable insights that will contribute to improving early diagnosis and outcomes for liver disease across high-risk populations.
We used NIHR Research Partnerships funding to develop a broad network of stakeholders based in the North West of England, an area with very high liver disease mortality but historically low research activity (Figure 1). Patient/public involvement and partnerships (alongside our PPIE partner Vocal) were embedded from the outset. In parallel an international group of academics and methodologists, including health economists, epidemiologists, decision-analysts with experience in liver disease research was developed. Using facilitated meetings and workshops, unmet needs were identified and research questions formed. Methods to address these challenges were explored and scrutinised in a final event and best projects to take forward for further funding selected. A framework outline of our approach is shown in Figure 2 and specific aims and objectives are outlined below.
Build a research network growing out of the expertise developed in Manchester to Greater Manchester and Lancashire.
Use national expertise in data access and curation and evaluation methodology to develop an ambitious and feasible research plan.
Establish links with local community organisations in underserved areas.
Development of fundable and implementable research proposal to answer an important unmet need in liver disease.
Patient and Public Involvement and Engagement (PPIE) collaboration was involved at every stage of project design and delivery. Led by specialist trained PPIE facilitators, five public contributors with lived experience of liver disease, or increased risk, were recruited and formed part of the core Project Steering Group (PSG) as Public Governance Advisors. Using an existing network of more than 100 public advisors (the Vocal Liver Network), PPIE facilitators and Public Governance Advisors connected with underserved areas and groups to canvas their viewpoints regarding liver disease. These qualitative learnings were presented back to the PSG to help inform decision making. Specific questions around perceived and experienced barriers in communities were explored. The PPIE team reviewed and fed into dissemination plans and co-delivered talks about the project and patient experience.
The project included five distinct work packages (WP) designed to achieve our aims and objectives:
WP1: Clinical network development.
WP2: Decision analyst network development.
WP3: Engagement with underserved communities.
WP4: Research proposal development.
WP5: Overall management.
We developed a clinical research network encompassing Greater Manchester and Lancashire (WP1) as the foundational structure for this liver disease research initiative. We identified new clinical members who were then vetted and integrated into the network, ensuring a collaborative alignment with the project's goals. Areas of very high disease prevalence were preferentially targeted.
A regional clinical network (WP1) and an international decision-analyst network (WP2) were developed separately but in parallel. The clinical network workshops focused on identifying unmet clinical needs in managing people with liver disease and the decision-analyst network was developed to ensure appropriate methodological expertise were in situ to develop a research proposal capable to address the clinical challenge.
In the second phase of the NWERP the two networks were amalgamated in a facilitated workshop in a Project Steering Group (PSG), drawing upon a diverse network of professionals to ensure representation across relevant disciplines. This interdisciplinary PSG formed the backbone of the NWERP and comprised 2 public governance advisors, persons with lived experience, regional representation of clinicians managing liver disease in primary and secondary care, alcohol nurses, substance use support workers, dietitians, statisticians, methodologists, health economists, and qualitative researchers. A mentorship program was also implemented to support the professional development of early-career researchers within the network. Mentorship focuses on skill-building and professional networking, ensuring that early-career researchers are well-equipped to become active contributors to liver disease research in the region.
To understand the clinical landscape of liver disease and identify priority research questions, we conducted monthly work package online meetings in addition to quarterly in-person meetings. Leveraging prior connections within the regional network, clinicians were invited to provide feedback on their experiences and perceptions related to liver disease management. These discussion results served as a foundation for identifying evidence gaps and formulating research questions to be addressed in WP4.
To further enrich the interdisciplinary expertise, a diverse group of stakeholders was invited to join. This group included senior clinicians, scientists, academics, allied health professionals, industry representatives, Public Governance Advisors, and individuals from the voluntary and charity sectors. Stakeholders were identified and invited through the mapping of research and clinical networks, as well as through personal and professional connections.
We used the PICO framework to formulate the evidence based, well-structured research questions. PICO (Population, Intervention, Comparison and Outcome), is a useful structure for framing and focusing clinical questions and ensuring that the research is clear, specific and measurable. PICO summaries for the final four topics were prepared and shared with all the stakeholders. Following the PICO distribution a Dragons’ Den event was organised to select project or projects to take forward towards an application.
A "Dragons’ Den" event for research questions was inspired by the popular television show Dragons’ Den, where entrepreneurs pitch their business ideas to a panel of investors (the "Dragons") to secure funding. In this research context, this format is used for presenting research proposals or ideas to a panel the Project Steering Group.
How it worked:
1. Research Teams or Individuals Present Proposals: Researchers pitch their project ideas or research questions in a clear and concise manner, outlining the objectives, methodology, potential outcomes, and impact.
2. Panel Feedback: A panel of experts (senior researchers, clinicians, health economists, implementation scientists, Public Governance Advisors, programme managers) questioned and challenged the presenters, providing constructive feedback. This was aimed at refining the research ideas and ensuring the proposed projects are feasible, impactful, and well-designed.
3. Decision-Making: Following the presentations and discussions, the panel voted on whether the research ideas should move forward for full-fledged development.
The Dragons’ Den format fosters critical thinking, clear communication, and collaboration in refining research ideas, enabling in-depth discussions that ensure high-quality outcomes. We adopted this approach to streamline the thought process and selection of topics, facilitating the development of robust research proposals.
The establishment of the clinical research network resulted in a cohesive group of multidisciplinary members actively collaborating to address the region’s liver disease research needs. Active engagement with regions with high disease burden and low disease activity was exemplified by most network members being new to clinical research. Monthly meetings facilitated open discussions, allowing new members to integrate and contribute meaningfully to network activities. The mentorship initiative focusing on early-career researchers gaining valuable insights and skills through structured guidance from established experts in the field.
The structured discussions gathered from online and in-person meetings provided insights from a diverse sample of stakeholders, with a high response rate achieved by leveraging existing network connections. Initial findings indicate a set of common challenges and knowledge gaps in the early detection and management of liver disease. These insights guided the formulation of research questions, aligning research priorities with the practical needs of clinicians managing liver disease on the front lines. Extensive discussion during monthly online meetings of stakeholders, including gastroenterology and hepatology clinicians, led to identification of the following areas of liver disease to develop the future research proposal:
An international Decision-Analyst Network was established. An expert panel meeting was held in July 2023 to bring together experts in decision-analytic and epidemiological modelling, health economics, risk-prediction and implementation science with an interest in liver disease. Collectively the expert panel discussed the current state of the evidence available, and key research areas needed, to inform the implementation of interventions along the spectrum of the liver care pathway. The decision analyst network was combined with the clinical network (including PPIE team) to develop the project steering group for WP4. This ensured from conceptualisation potential projects were with best research approaches, methodologically robust, economically viable, and grounded in the real-world clinical context.
A key failure of a large liver research projects has been lacking involvement of decision analysts at conceptual stages of research. We have established NRRP, and assembled a team of Health Economist (Katherine Payne, UoM), Implementation scientist (Gabriel Rogers, UoM), all collaborating with clinical experts to develop an evidence-based research proposal targeting early detection of liver disease.
The project fostered close collaboration with Greater Manchester’s Health Innovation Network: Health Innovation Manchester (HInM). As a project partner, HInM facilitated connections with relevant stakeholders, from healthcare providers to regional decision-makers, supporting a framework for rapid translation and adoption of research outcomes. This partnership enhances the project’s visibility and promotes readiness for large-scale application of any developed technologies or interventions, ultimately aiming for impactful improvements in liver disease management across Northwest England.
We established a PPIE panel of Public Governance Advisors who remain actively engaged throughout the project. Their consistent input across scheduled meetings ensured that the proposal reflects the experiences, challenges, and priorities of individuals at risk of liver disease, as well as their families and carers.
We discussed and gathered comprehensive feedback on barriers experienced by unique communities, alongside strategies for effective communication and dissemination. This feedback helped shape the proposal’s design and inform approaches to future studies, ensuring they are sensitive to the needs of target populations and accessible to all community members. Our PPIE contributors were instrumental in the design of the project and our public co-applicant helped develop the ‘Plain English Summary’.
The outcome of this project is a co-designed research proposal that is representative of and acceptable to the target population and geography. By embedding community perspective, this partnership produced a proposal directly aligned with the needs of those affected by liver disease.
Research proposal development was undertaken in a structured process, with facilitators chosen for expertise dependent on the point of the process. Initially specific evidence gaps in liver disease research were identified. Using data from pre-meeting clinical experience survey, we assessed current challenges and limitations in liver disease management. Evidence gaps identified were then scrutinised for research questions in a facilitated workshop, bringing together clinicians, public contributors, methodological expertise and other stakeholders. In the final workshop, we formulated specific research proposals and defined the evidence required to address them.
This discussion took place in an in-person decision analytical meeting, attended by clinicians, health economists, methodologists and implementation scientists, who assessed the feasibility, cost-effectiveness, and potential impact of proposed interventions using decision analytics.
At the end of the process, four potential projects were created, as shown in Table 1. These projects were then presented and judged in the final workshop that we named the Dragons’ Den event to generate interest and attendance.
MASLD (metabolic dysfunction associated liver disease), MetALD (metabolic and alcohol associated liver disease), QoL (quality of life).
Detailed PICO notes of all the above-mentioned four topics (Table 1) were prepared and shared with all stakeholders before the event. All topics had a dedicated presenter and were given 20 minutes for presentation and discussion. All 24 attendees were able to vote using a structured scoring sheet (Table 2). Figure 3 summarises the stakeholders present during the event and their voting by project topic (Figure 4).
The clinical research team presented each topic followed by in-depth discussion. Each proposal’s alignment with NIHR priorities, financial feasibility, technical achievability, relevance to patient and the public and its ability to address a clinical need was assessed in a structured format.
Comments and suggestions received for all PICO topics were summarised, including barriers to overcome and potential protocol consideration. None of the projects were unanimously favoured by all the stakeholders as indicated by the score calculation (Figure 4). However, when individual votes were considered, Topic 1 received 38%, Topic 2 received 56% and Topic 4 received 6%. Topic 3 received no vote (Figure 5).
The Northwest England Research Partnership (NWERP) has made progress in addressing the rising burden of liver disease in the region. By fostering collaboration, involving public contributors, and leveraging expertise in data access and curation and evaluation methodologies, the partnership has laid a strong foundation for future research on the diagnosis and management of liver diseases.
A key strength of the NWERP has been its commitment to patient and public involvement (PPIE). By actively engaging with people at increased risk of liver disease, patients, carers, and community members, the partnership has ensured that research priorities are aligned with real-world needs.
To address the growing global burden of liver disease, broad spectrum partnership is essential. By sharing knowledge, resources, and expertise, researchers can accelerate progress in this field. The NWERP established these partnerships across the North West of England to foster the research initiatives and aims to submit the most promising proposals to the relevant national research streams.
No immediate ethical concerns are associated with this partnership project or the networking events and workshops. Since this initiative did not constitute formal research, workshop participants engaged as partnership members to share insights, perspectives, and ideas.
Athwal, Varinder (2025). North West England Research Partnership Data. University of Manchester. Dataset. https://doi.org/10.48420/308626914
This project contains the following underlying data:
Data file 1: PICO Figures and data file
Data file 2: PICO Score sheets for all attendees.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Register with NIHR Open Research
Already registered? Sign in
If you are a previous or current NIHR award holder, sign up for information about developments, publishing and publications from NIHR Open Research.
We'll keep you updated on any major new updates to NIHR Open Research
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)